当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Syntheses of Scabrolide B, Ineleganolide, and Related Norcembranoids
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-20 , DOI: 10.1021/jacs.4c16629 Emma J. Simmons, David B. Ryffel, Diego A. Lopez, Yaroslav D. Boyko, David Sarlah
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-20 , DOI: 10.1021/jacs.4c16629 Emma J. Simmons, David B. Ryffel, Diego A. Lopez, Yaroslav D. Boyko, David Sarlah
|
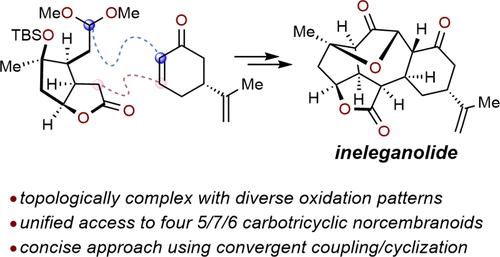
Concise total syntheses of several 5/7/6 norcembranoids, including ineleganolide, scabrolide B, sinuscalide C, and fragilolide A have been achieved through a fragment coupling/ring closure approach. The central seven-membered ring was forged through sequential Mukaiyama–Michael/aldol reactions using norcarvone and a decorated bicyclic lactone incorporating a latent electrophile. Subsequent manipulations installed the reactive enedione motif and delivered scabrolide B in 11 steps from a chiral pool-derived enone. Finally, ineleganolide, sinuscalide C, and fragilolide A were each accessed in one additional step.
更新日期:2024-12-20






























 京公网安备 11010802027423号
京公网安备 11010802027423号