当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Talarergosteroids A–C: Three Unusual Steroid-Polyketone Conjugates with Antifungal Activity from a Kandelia Obovata Derived Fungus Talaromyces sp
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-12-19 , DOI: 10.1021/acs.jafc.4c10156 Jialin Li, Zirong Lin, Haiqi Zeng, Jiechang Zeng, Siyao Ye, Chen Chen, Hao Jia, Kang Li, Zhigang She, Yuhua Long
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-12-19 , DOI: 10.1021/acs.jafc.4c10156 Jialin Li, Zirong Lin, Haiqi Zeng, Jiechang Zeng, Siyao Ye, Chen Chen, Hao Jia, Kang Li, Zhigang She, Yuhua Long
|
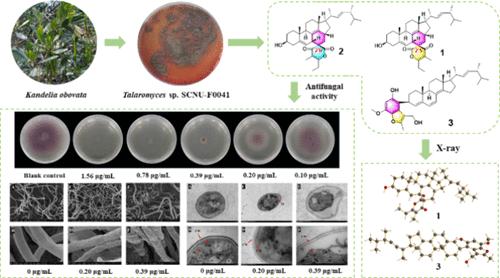
Three previously undescribed steroid–polyketone conjugates, talarergosteroids A–C (1–3), together with talarergosteroid D (4), which was first identified from a natural source, were isolated from a Kandelia Obovata derived fungus Talaromyces sp. SCNU-F0041. Compounds 1 and 2 bear a complicated 6/6/6/5/6/6 hexacyclic ring system characterized by an oxaspiro[5.5]undecane architecture. Compound 3 possesses a benzofuran moiety substituted at C-3 in ergosterol. The structures of the new compounds were identified by comprehensive spectroscopic analysis, X-ray diffraction, and electronic circular dichroism (ECD) calculation. Talarergosteroid B (2) showed significant inhibitory activity against the agricultural plant pathogen Fusarium oxysporum f. sp. lycopersici (MIC = 0.78 μg/mL), outperforming the positive control carbendazim (MIC = 1.56 μg/mL). Preliminary research disclosed that compound 2 may inhibit the spore germination progress, malform the fungal mycelium, and damage the organelle. These results indicate that compound 2 could be a potential fungicidal lead compound against Fusarium oxysporum f. sp. lycopersici.
更新日期:2024-12-20






























 京公网安备 11010802027423号
京公网安备 11010802027423号