当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Promoted *OH Adsorption Facilitates C–C Bond Cleavage for Efficient Electrochemical Upcycling of Polyethylene Terephthalate
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-20 , DOI: 10.1021/acscatal.4c05352 Jinyong Sun, Binkai Shi, Shuixing Dai, Lei Chu, Huanlei Wang, Minghua Huang
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-20 , DOI: 10.1021/acscatal.4c05352 Jinyong Sun, Binkai Shi, Shuixing Dai, Lei Chu, Huanlei Wang, Minghua Huang
|
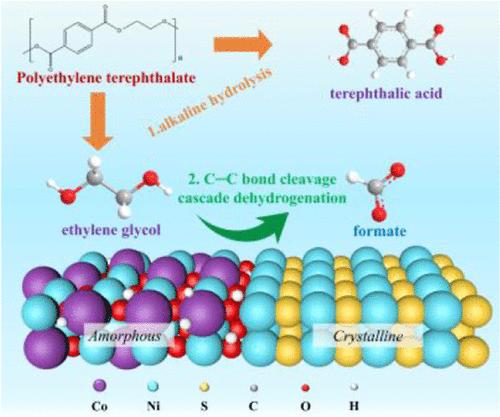
The electrochemical oxidation of ethylene glycol (EG) derived from polyethylene terephthalate (PET) plastic into value-added chemicals, coupled with hydrogen evolution, offers a promising approach to addressing plastic pollution. However, the mechanisms by which the adsorption of key reaction intermediates affects the EG oxidation reaction (EGOR) are not well understood. To investigate this, we synthesized two model catalysts: amorphous-phase CoNiOOH/NF and CoNiOOH–Ni3S2/NF with an amorphous/crystalline interface. Detailed characterizations and theoretical calculations demonstrate that the amorphous/crystalline interface in CoNiOOH–Ni3S2/NF shifts the d-band center upward, enhancing the adsorption of EG and *OH compared to amorphous CoNiOOH/NF. Enhanced *OH adsorption is crucial for promoting C–C bond cleavage and subsequent dehydrogenation. In situ electrochemical infrared absorption spectroscopy (IRAS) and theoretical calculations reveal that formate (FA) is primarily formed through cleavage of the C–C bond in glycolic acid, followed by oxidation. Notably, CoNiOOH–Ni3S2/NF achieves industrial-level current densities of 500 mA cm–2 at an ultralow potential of 1.45 V vs RHE, with a Faradaic efficiency (FE) of 96.6% and FA productivity of 3.14 mmol cm–2 h–1 at 1.70 V vs RHE. This study offers valuable insights for designing efficient heterojunction catalysts for the electrochemical upcycling of PET plastics.
更新日期:2024-12-20






























 京公网安备 11010802027423号
京公网安备 11010802027423号