当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Axially Chiral Vinyl Halides via Cu(I)-Catalyzed Enantioselective Radical 1,2-Halofunctionalization of Terminal Alkynes
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-20 , DOI: 10.1021/acscatal.4c06672 Jun-Bin Tang, Jun-Qian Bian, Zhihan Zhang, Yong-Feng Cheng, Li Qin, Qiang-Shuai Gu, Peiyuan Yu, Zhong-Liang Li, Xin-Yuan Liu
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-20 , DOI: 10.1021/acscatal.4c06672 Jun-Bin Tang, Jun-Qian Bian, Zhihan Zhang, Yong-Feng Cheng, Li Qin, Qiang-Shuai Gu, Peiyuan Yu, Zhong-Liang Li, Xin-Yuan Liu
|
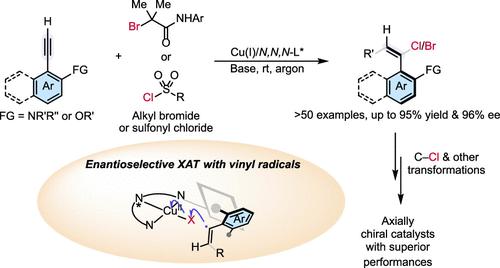
Organohalides are crucial in modern organic synthesis, thanks to their robust and versatile reactivity in cross-coupling and other key transformations. However, catalytic asymmetric methods for producing enantioenriched organohalides, particularly axially chiral vinyl halides, remain underdeveloped. Here, we present a Cu(I)-catalyzed, highly enantioselective radical alkyne 1,2-halofunctionalization, utilizing custom-designed tridentate anionic N,N,N-ligands with bulky peripheral substituents. This method efficiently employs (hetero)aryl and alkyl sulfonyl chlorides, as well as α-carbonyl alkyl bromides, as radical precursors and utilizes a diverse range of 2-amino and 2-oxy aryl terminal alkynes as substrates to produce highly enantioenriched axially chiral vinyl halides. The reaction is scalable to gram quantities, and the vinyl halides can be further transformed into axially chiral thiourea, pyridyl carboxamide, and quinolyl sulfonamide compounds, some of which show significant potential in asymmetric catalysis. Both experimental and theoretical mechanistic studies support an enantioselective halogen atom transfer mechanism. This method opens an avenue for accessing axially chiral organohalides, facilitating their broad applications in various related fields.
更新日期:2024-12-20






























 京公网安备 11010802027423号
京公网安备 11010802027423号