当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlling Co 3d/O 2p Orbital Hybridization in LaCoO3 by Modulating the Co–O–Co Bond Angle for Enhanced Oxygen Evolution Reaction Catalysis
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-19 , DOI: 10.1021/acscatal.4c05479 Baoxin Ge, Pengyang Jiang, Biyi Chen, Caijin Huang
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-12-19 , DOI: 10.1021/acscatal.4c05479 Baoxin Ge, Pengyang Jiang, Biyi Chen, Caijin Huang
|
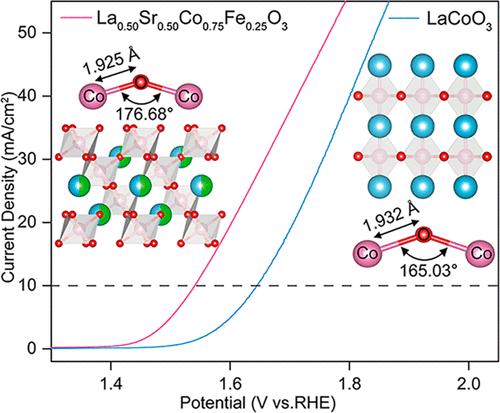
The orbital hybridization between metal and oxygen of perovskite catalysts can lower the overpotential and enhance the oxygen evolution reaction (OER) activity. This study combines density functional theory with experiments to clarify how Sr/Fe codoping modulates orbital hybridization and enhances OER catalytic activity of LaCoO3. The as-prepared La0.50Sr0.50Co0.75Fe0.25O3 shows remarkable performance with a low overpotential of 310 mV at 10 mA cm–2 current density and a 107.03 mV dec–1 Tafel slope, outperforming most state-of-the-art perovskite-based OER electrocatalysts. The experimental results confirm that Sr/Fe codoping enhances the expansion of Co–O–Co bond angles and strengthens the covalency of the Co–O bond in LaCoO3, leading to enhanced electrocatalytic activity. Moreover, increasing Sr doping reduces the distance between the Co 3d/O 2p center and the Fermi level, decreasing the energy difference between them and enhancing the degree of orbital hybridization between Co 3d and O 2p. As the degree of Co 3d/O 2p orbital hybridization increases, a higher charge transfer was found between the active center and intermediate product, OOH, reducing the energy barrier of the rate-determining step while lowering the overpotential. This study provides thorough insight into the rational design of OER catalysts based on orbital hybridization.
更新日期:2024-12-20






























 京公网安备 11010802027423号
京公网安备 11010802027423号