当前位置:
X-MOL 学术
›
Gastroenterology
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
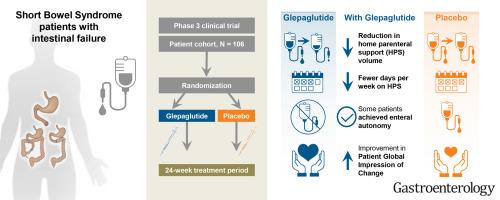
Glepaglutide, a Long-acting Glucagon-like Peptide-2 Analog, Reduces Parenteral Support in Patients with Short Bowel Syndrome: a Phase 3, Randomized, Controlled Trial
Gastroenterology ( IF 25.7 ) Pub Date : 2024-12-19 , DOI: 10.1053/j.gastro.2024.11.023 Palle B. Jeppesen, Tim Vanuytsel, Sukanya Subramanian, Francisca Joly, Geert Wanten, Georg Lamprecht, Marek Kunecki, Farooq Rahman, Thor S.S. Nielsen, Mark Berner-Hansen, Ulrich-Frank Pape, David F. Mercer
更新日期:2024-12-20
Gastroenterology ( IF 25.7 ) Pub Date : 2024-12-19 , DOI: 10.1053/j.gastro.2024.11.023 Palle B. Jeppesen, Tim Vanuytsel, Sukanya Subramanian, Francisca Joly, Geert Wanten, Georg Lamprecht, Marek Kunecki, Farooq Rahman, Thor S.S. Nielsen, Mark Berner-Hansen, Ulrich-Frank Pape, David F. Mercer
|






























 京公网安备 11010802027423号
京公网安备 11010802027423号