当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single-cell RNA seq-derived signatures define response patterns to atezolizumab + bevacizumab in advanced hepatocellular carcinoma
Journal of Hepatology ( IF 26.8 ) Pub Date : 2024-12-19 , DOI: 10.1016/j.jhep.2024.12.016 Sarah Cappuyns, Marta Piqué-Gili, Roger Esteban-Fabró, Gino Philips, Ugne Balaseviciute, Roser Pinyol, Albert Gris-Oliver, Vincent Vandecaveye, Jordi Abril-Fornaguera, Carla Montironi, Laia Bassaganyas, Judit Peix, Marcus Zeitlhoefler, Agavni Mesropian, Júlia Huguet-Pradell, Philipp K. Haber, Igor Figueiredo, Giorgio Ioannou, Edgar Gonzalez-Kozlova, Antonio D’Alessio, Josep M. Llovet
中文翻译:

单细胞 RNA seq 衍生的特征定义了晚期肝细胞癌对 atezolizumab + 贝伐珠单抗的反应模式
atezolizumab 和贝伐珠单抗 (atezo+bev) 的组合是目前晚期肝细胞癌 (HCC) 的治疗标准,中位总生存期 (OS) 为 19.2 个月。在这里,我们旨在揭示驱动临床获益与 atezo + bev 耐药性的潜在细胞过程。
我们利用晚期 HCC 中单细胞 RNA 测序的力量来获得概括 21 种细胞表型的基因表达特征。这些特征应用于接受 atezo + bev (n=317) 与 atezolizumab (n=47) 或索拉非尼 (n=58) 治疗的晚期 HCC 患者的 422 个 RNA 测序样本作为对照。
我们揭示了对 atezo + bev 的两种不同的反应模式。首先,免疫介导的反应以 CD8+ T 效应细胞和促炎性 CXCL10+ 巨噬细胞的组合存在为特征,代表免疫丰富的微环境。其次,非免疫性、血管生成相关反应可通过 VEGF 辅助受体神经纤毛蛋白-1 (NRP1) 的表达降低来区分,NRP1 是一种生物标志物,可特异性预测 atezo + bev 与索拉非尼相比 OS 改善 (p = 0.039)。原发性耐药与免疫抑制性髓系细胞群(即 CD14 + 单核细胞和 TREM2 + 巨噬细胞)的富集以及 Notch 通路激活有关。基于这些机制见解,我们定义了“免疫能力”和“血管生成驱动”分子亚群,每个分子亚群都与 atezo + bev 与索拉非尼相比明显更长的 OS 相关(相互作用 p = 0.027),以及“耐药”亚群。
我们的研究揭示了 atezolizumab 联合贝伐珠单抗在晚期 HCC 中临床益处的两个不同的分子亚群(“免疫功能正常”和“血管生成驱动”)以及对这种疗法的原发性耐药的主要特征,从而提供了一个分子框架,可以根据临床结果对患者进行分层,并指导克服耐药性的潜在策略。
更新日期:2024-12-20
Journal of Hepatology ( IF 26.8 ) Pub Date : 2024-12-19 , DOI: 10.1016/j.jhep.2024.12.016 Sarah Cappuyns, Marta Piqué-Gili, Roger Esteban-Fabró, Gino Philips, Ugne Balaseviciute, Roser Pinyol, Albert Gris-Oliver, Vincent Vandecaveye, Jordi Abril-Fornaguera, Carla Montironi, Laia Bassaganyas, Judit Peix, Marcus Zeitlhoefler, Agavni Mesropian, Júlia Huguet-Pradell, Philipp K. Haber, Igor Figueiredo, Giorgio Ioannou, Edgar Gonzalez-Kozlova, Antonio D’Alessio, Josep M. Llovet
|
Background & Aims
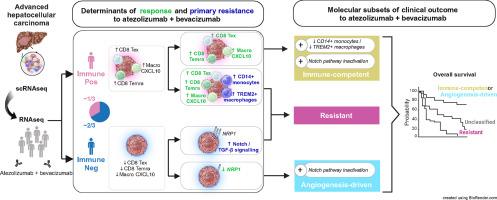
The combination of atezolizumab and bevacizumab (atezo+bev) is the current standard of care for advanced hepatocellular carcinoma (HCC), providing a median overall survival (OS) of 19.2 months. Here, we aim to uncover the underlying cellular processes driving clinical benefit versus resistance to atezo+bev.Methods
We harnessed the power of single-cell RNA sequencing in advanced HCC to derive gene expression signatures recapitulating 21 cell phenotypes. These signatures were applied to 422 RNA-sequencing samples of advanced HCC patients treated with atezo+bev (n=317) versus atezolizumab (n=47) or sorafenib (n=58) as comparators.Results
We unveiled two distinct patterns of response to atezo+bev. First, an immune-mediated response characterized by the combined presence of CD8+ T effector cells and pro-inflammatory CXCL10+ macrophages, representing an immune rich microenvironment. Second, a non-immune, angiogenesis-related response distinguishable by a reduced expression of the VEGF co-receptor neuropilin-1 (NRP1), a biomarker that specifically predicts improved OS upon atezo+bev vs sorafenib (p = 0.039). Primary resistance was associated with an enrichment of immunosuppressive myeloid populations, namely CD14+ monocytes and TREM2+ macrophages, and Notch pathway activation. Based on these mechanistic insights we define "Immune-competent" and "Angiogenesis-driven" molecular subgroups, each associated with a significantly longer OS with atezo+bev versus sorafenib (p of interaction = 0.027), and a “Resistant” subset.Conclusion
Our study unveils two distinct molecular subsets of clinical benefit to atezolizumab plus bevacizumab in advanced HCC (“Immune-competent” and “Angiogenesis-driven”) as well as the main traits of primary resistance to this therapy, thus providing a molecular framework to stratify patients based on clinical outcome and guiding potential strategies to overcome resistance.中文翻译:

单细胞 RNA seq 衍生的特征定义了晚期肝细胞癌对 atezolizumab + 贝伐珠单抗的反应模式
背景和目标
atezolizumab 和贝伐珠单抗 (atezo+bev) 的组合是目前晚期肝细胞癌 (HCC) 的治疗标准,中位总生存期 (OS) 为 19.2 个月。在这里,我们旨在揭示驱动临床获益与 atezo + bev 耐药性的潜在细胞过程。
方法
我们利用晚期 HCC 中单细胞 RNA 测序的力量来获得概括 21 种细胞表型的基因表达特征。这些特征应用于接受 atezo + bev (n=317) 与 atezolizumab (n=47) 或索拉非尼 (n=58) 治疗的晚期 HCC 患者的 422 个 RNA 测序样本作为对照。
结果
我们揭示了对 atezo + bev 的两种不同的反应模式。首先,免疫介导的反应以 CD8+ T 效应细胞和促炎性 CXCL10+ 巨噬细胞的组合存在为特征,代表免疫丰富的微环境。其次,非免疫性、血管生成相关反应可通过 VEGF 辅助受体神经纤毛蛋白-1 (NRP1) 的表达降低来区分,NRP1 是一种生物标志物,可特异性预测 atezo + bev 与索拉非尼相比 OS 改善 (p = 0.039)。原发性耐药与免疫抑制性髓系细胞群(即 CD14 + 单核细胞和 TREM2 + 巨噬细胞)的富集以及 Notch 通路激活有关。基于这些机制见解,我们定义了“免疫能力”和“血管生成驱动”分子亚群,每个分子亚群都与 atezo + bev 与索拉非尼相比明显更长的 OS 相关(相互作用 p = 0.027),以及“耐药”亚群。
结论
我们的研究揭示了 atezolizumab 联合贝伐珠单抗在晚期 HCC 中临床益处的两个不同的分子亚群(“免疫功能正常”和“血管生成驱动”)以及对这种疗法的原发性耐药的主要特征,从而提供了一个分子框架,可以根据临床结果对患者进行分层,并指导克服耐药性的潜在策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号