当前位置:
X-MOL 学术
›
Sep. Purif. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tunable Ca doping in MgFeMnO4 electrode for enhanced hardness removal selectivity
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-12-20 , DOI: 10.1016/j.seppur.2024.131215 Yue Zhu, Pengfei Nie, Jianan Sun, Boshuang Zhang, Jianmao Yang, Jianxin Guo, Jianyun Liu
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-12-20 , DOI: 10.1016/j.seppur.2024.131215 Yue Zhu, Pengfei Nie, Jianan Sun, Boshuang Zhang, Jianmao Yang, Jianxin Guo, Jianyun Liu
|
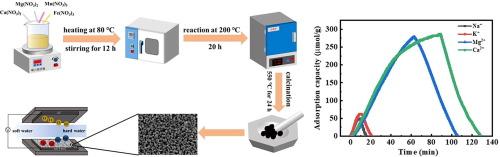
Control of water hardness, primarily caused by Ca2+, Mg2+, is critical for ensuring human health, safe industrial production and environmental protection. Recently, capacitive deionization (CDI) has attracted much attention in water purification. However, conventional carbon-based electrodes face challenges in hardness removal due to low selectivity to Ca2+ and Mg2+. This study introduces a novel spinel polymetallic oxide electrode material, Mg1-xCaxFeMnO4 (x = 0, 0.5, 1), synthesized via a facile sol–gel method, for hardness removal. The Mg1-xCaxFeMnO4 electrode demonstrated remarkable electrochemical specific capacity and enhanced selective adsorption of hardness ions through Mn/Fe redox reaction accompanied with ion intercalation/deintercalation. Especially, the Ca content in Mg1-xCaxFeMnO4 significantly influences its crystal structure and electrochemical properties, with Mg0.5Ca0.5FeMnO4 exhibiting optimal electrochemical activity and high specific capacity. In a CDI device, the Mg0.5Ca0.5FeMnO4 electrode delivered exceptional adsorption capacities for Mg2+ (278.5 μmol/g) and Ca2+ (287.1 μmol/g), significantly surpassing those for Na+ (41.1 μmol/g) and K+ (45.1 μmol/g), demonstrating its selective adsorption to hardness ions. The Mg0.5Ca0.5FeMnO4-based CDI system was successfully applied for actual groundwater softening, with hardness removal ratio of over 80 %. Additionally, the electrode maintained superior structural stability over 100 adsorption/desorption cycles. The selective adsorption mechanisms of ions on multi-metal oxides were investigated by X-ray diffraction, X-ray photo spectroscopy and electrochemical characterization, and further confirmed by density functional theory calculation. This research provides valuable insights into the design of advanced materials for efficient and selective water softening in industrial applications.
更新日期:2024-12-20






























 京公网安备 11010802027423号
京公网安备 11010802027423号