当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Chlorozincate Anion Intercalation into Layered Carbon Materials for the Cathodes of Aqueous Zinc Metal Secondary Batteries
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-12-20 , DOI: 10.1021/acs.chemmater.4c02551 Akane Inoo, Junichi Inamoto, Koji Nakanishi, So Fujinami, Yoshiaki Matsuo
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-12-20 , DOI: 10.1021/acs.chemmater.4c02551 Akane Inoo, Junichi Inamoto, Koji Nakanishi, So Fujinami, Yoshiaki Matsuo
|
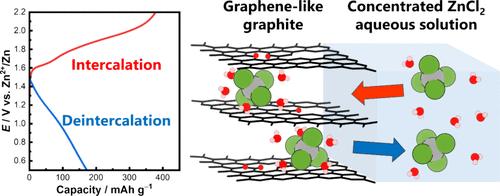
Aqueous zinc metal secondary batteries (ZSBs) are expected to be next-generation secondary batteries, and it is important to explore cathode materials and electrolyte solutions that exhibit excellent electrochemical properties for their practical use. In this study, we employed a layered carbon material named graphene-like graphite (GLG) as a cathode active material and a concentrated aqueous zinc chloride solution as an electrolyte solution, and its electrochemical anion intercalation reaction was investigated. As a result, GLG obtained at 300 °C of thermal treatment (GLG300) exhibited lower anion intercalation potential and better Coulombic efficiency in ZnCl2·2.33H2O compared to graphite and GLG obtained at 700 °C. X-ray diffraction measurement suggested that GLG300 formed a stage-1 intercalation compound at 1.8 V vs Zn2+/Zn, and extended X-ray absorption fine structure analysis revealed that the intercalated anion was hydrated [ZnCl4]2–. The initial discharge capacity of GLG300 was approximately 170 mAh g–1 in the potential range of 0.5–2.2 V with a current density of 20 mA g–1. The charge–discharge cycling test showed that GLG300 had good reversibility, the discharge capacity remained above 110 mAh g–1, and the Coulombic efficiency approached nearly 100% at the 50th cycle. These results demonstrated that the system using GLG300 and concentrated aqueous zinc chloride solution exhibits excellent cathode properties as aqueous ZSBs and showed great promise for their future practical use.
更新日期:2024-12-20






























 京公网安备 11010802027423号
京公网安备 11010802027423号