当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Dual Catalytic Approach for the Halogen-Bonding-Mediated Reductive Cleavage of α-Bromodifluoroesters and Amides
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-12-19 , DOI: 10.1021/acs.joc.4c02413 Tarannum Tasnim, Negin Shafiei, Katelyn J. Laminack, Bailey S. Robertson, Nash E. Nevels, Christopher J. Fennell, Spencer P. Pitre
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-12-19 , DOI: 10.1021/acs.joc.4c02413 Tarannum Tasnim, Negin Shafiei, Katelyn J. Laminack, Bailey S. Robertson, Nash E. Nevels, Christopher J. Fennell, Spencer P. Pitre
|
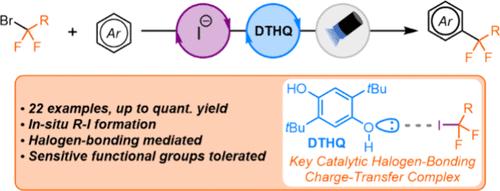
While charge-transfer complexes involving halogen-bonding interactions have emerged as an alternative strategy for the photogeneration of carbon radicals, examples using (fluoro)alkyl bromides are limited. This report describes a dual catalytic approach for radical generation from α-bromodifluoroesters and amides under visible-light irradiation. Mechanistic studies suggest that the reaction proceeds through in situ bromide displacement using a catalytic iodide salt, generating a C–I bond that can be engaged by our halogen-bonding photocatalysis platform.
中文翻译:

卤素键介导的 α-溴二氟酯和酰胺还原裂解的双重催化方法
虽然涉及卤素键相互作用的电荷转移络合物已成为光生碳自由基的替代策略,但使用(氟)烷基溴的例子是有限的。本报告描述了一种在可见光照射下从 α-溴二氟酯和酰胺产生自由基的双重催化方法。机理研究表明,反应通过使用催化碘盐的原位溴化物置换进行,产生 C-I 键,该键可以通过我们的卤素键合光催化平台参与。
更新日期:2024-12-19
中文翻译:

卤素键介导的 α-溴二氟酯和酰胺还原裂解的双重催化方法
虽然涉及卤素键相互作用的电荷转移络合物已成为光生碳自由基的替代策略,但使用(氟)烷基溴的例子是有限的。本报告描述了一种在可见光照射下从 α-溴二氟酯和酰胺产生自由基的双重催化方法。机理研究表明,反应通过使用催化碘盐的原位溴化物置换进行,产生 C-I 键,该键可以通过我们的卤素键合光催化平台参与。






























 京公网安备 11010802027423号
京公网安备 11010802027423号