当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electroredox N-Heterocyclic Carbene-Catalyzed Enantioselective (3 + 3) Annulation of Enals with 2-Naphthols
Organic Letters ( IF 4.9 ) Pub Date : 2024-12-19 , DOI: 10.1021/acs.orglett.4c03879 Vikas Kale, Sayan Shee, Shiv Dutt, Nidhi Sinha, Akkattu T. Biju, Prabal Banerjee
Organic Letters ( IF 4.9 ) Pub Date : 2024-12-19 , DOI: 10.1021/acs.orglett.4c03879 Vikas Kale, Sayan Shee, Shiv Dutt, Nidhi Sinha, Akkattu T. Biju, Prabal Banerjee
|
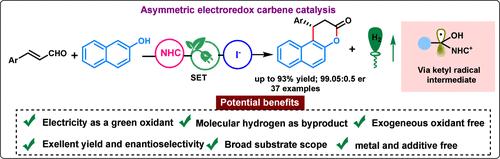
Developing asymmetric transformations using electroredox and N-heterocyclic carbene (NHC)-catalyzed radical pathways is still desirable and challenging. Herein, we report an iodide-promoted β-carbon activation (LUMO-lowering process) of enals via electroredox carbene catalysis coupled with a hydrogen evolution reaction (HER). This strategy offers an environmentally friendly and sustainable route for rapidly assembling synthetically useful chiral naphthopyran-3-one in good to excellent yield and enantioselectivity using traceless electrons as inexpensive and greener oxidants. The mechanistic studies and cyclic voltammetry suggest that the reaction proceeds via direct single electron transfer (SET) of the in situ-generated Breslow intermediate.
中文翻译:

电氧化还原 N-杂环卡宾催化的对映选择性 (3 + 3) 烯醛与 2-萘酚的环化
使用电氧化还原和 N-杂环卡宾 (NHC) 催化的自由基途径开发不对称转化仍然是可取的和具有挑战性的。在此,我们报道了一种通过电氧化还原卡宾催化与析氢反应 (HER) 耦合的 烯醛 β 促进的 烯醛 碳活化(LUMO 降低过程)。该策略提供了一种环保且可持续的路线,使用无痕电子作为廉价且更环保的氧化剂,以良好到优异的产率和对映选择性快速组装合成有用的手性萘吡喃-3-酮。机理研究和循环伏安法表明,反应通过原位生成的 Breslow 中间体的直接单电子转移 (SET) 进行。
更新日期:2024-12-19
中文翻译:

电氧化还原 N-杂环卡宾催化的对映选择性 (3 + 3) 烯醛与 2-萘酚的环化
使用电氧化还原和 N-杂环卡宾 (NHC) 催化的自由基途径开发不对称转化仍然是可取的和具有挑战性的。在此,我们报道了一种通过电氧化还原卡宾催化与析氢反应 (HER) 耦合的 烯醛 β 促进的 烯醛 碳活化(LUMO 降低过程)。该策略提供了一种环保且可持续的路线,使用无痕电子作为廉价且更环保的氧化剂,以良好到优异的产率和对映选择性快速组装合成有用的手性萘吡喃-3-酮。机理研究和循环伏安法表明,反应通过原位生成的 Breslow 中间体的直接单电子转移 (SET) 进行。






























 京公网安备 11010802027423号
京公网安备 11010802027423号