当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
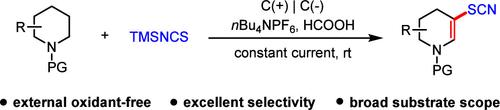
Electrochemical Desaturation and β-Thiocyanation of Cyclic Amides
Organic Letters ( IF 4.9 ) Pub Date : 2024-12-19 , DOI: 10.1021/acs.orglett.4c04141 Kaili Miao, Jin Zhang, Jiaxin Chen, Lin-Bao Zhang, Ming Li, Lirong Wen, Weisi Guo
Organic Letters ( IF 4.9 ) Pub Date : 2024-12-19 , DOI: 10.1021/acs.orglett.4c04141 Kaili Miao, Jin Zhang, Jiaxin Chen, Lin-Bao Zhang, Ming Li, Lirong Wen, Weisi Guo
|
The site-selective functionalization of cyclic amides provides an attractive protocol for the synthesis of valuable molecules. We report herein an electrochemical desaturation and β-thiocyanation of cyclic amides under external oxidant-free conditions. This method exhibits broad functional group tolerance, excellent selectivity, mild reaction conditions and can be applied for late-stage functionalization of bioactive molecules. Mechanistic studies indicate that an enamide intermediate might be involved.
中文翻译:

环酰胺的电化学去饱和和 β-硫氰化
环酰胺的位点选择性官能化为合成有价值的分子提供了一种有吸引力的方案。我们在此报道了环酰胺在无外部氧化剂条件下的电化学去饱和和β-硫代氰化反应。该方法表现出广泛的官能团耐受性、优异的选择性、温和的反应条件,可用于生物活性分子的后期官能团化。机理研究表明,可能涉及烯酰胺中间体。
更新日期:2024-12-19
中文翻译:

环酰胺的电化学去饱和和 β-硫氰化
环酰胺的位点选择性官能化为合成有价值的分子提供了一种有吸引力的方案。我们在此报道了环酰胺在无外部氧化剂条件下的电化学去饱和和β-硫代氰化反应。该方法表现出广泛的官能团耐受性、优异的选择性、温和的反应条件,可用于生物活性分子的后期官能团化。机理研究表明,可能涉及烯酰胺中间体。






























 京公网安备 11010802027423号
京公网安备 11010802027423号