当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocatalytic Generation of β-Fluoroalkylated α-Carbonyl Carbocations
Organic Letters ( IF 4.9 ) Pub Date : 2024-12-18 , DOI: 10.1021/acs.orglett.4c04273 Chong-Jin Zhang, Zhen-Zhen Liu, Yan-Biao Kang, Jian-Ping Qu
Organic Letters ( IF 4.9 ) Pub Date : 2024-12-18 , DOI: 10.1021/acs.orglett.4c04273 Chong-Jin Zhang, Zhen-Zhen Liu, Yan-Biao Kang, Jian-Ping Qu
|
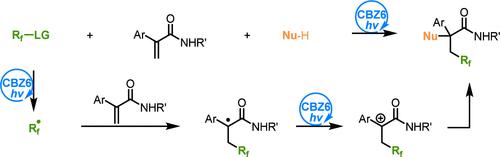
Nucleophilic addition to α,β-unsaturated carbonyl compounds normally occurs at the carbonyl carbon or β-carbon. The direct α-nucleophilic addition at the α-carbon can hardly be achieved due to electronic mismatch. In this work, we report the nucleophilic addition of β-fluoroalkyl α-carbonyl carbocations that are prepared via CBZ6-induced redox-neutral photocatalysis. In this process, the photocatalytic oxidation of the β-fluoroalkyl α-carbonyl radical to the corresponding carbocation is the key step. The β-fluoroalkyl α-carbonyl radical is generated in situ by the addition of a polyfluoroalkyl radical, which is generated by the photocatalytic fragmentation of polyfluoroalkyl sulfonyl chloride, to α,β-unsaturated carbonyls. The high E00 value of CBZ6 (3.19 V vs the saturated calomel electrode), which corresponds with the absorbed photoenergy, contributes to the high catalytic reactivity.
中文翻译:

光催化生成 β-氟烷基化 α-羰基碳化
α,β-不饱和羰基化合物的亲核加成通常发生在羰基碳或β-碳处。由于电子失配,几乎无法在 α-carbon 处实现直接的亲α加成。在这项工作中,我们报道了通过 CBZ6 诱导的氧化还原中性光催化制备的 β-氟烷基 α-羰基碳阳离子的亲核加成反应。在这个过程中,β-氟烷基α-羰基自由基的光催化氧化成相应的碳阳离子是关键步骤。β-氟烷基α-羰基自由基是通过向 α,β-不饱和羰基加成多氟烷基自由基而-羰基自由基在原位生成的。CBZ6 的高 E00 值(3.19 V 与饱和甘汞电极)对应于吸收的光能,有助于高催化反应性。
更新日期:2024-12-19
中文翻译:

光催化生成 β-氟烷基化 α-羰基碳化
α,β-不饱和羰基化合物的亲核加成通常发生在羰基碳或β-碳处。由于电子失配,几乎无法在 α-carbon 处实现直接的亲α加成。在这项工作中,我们报道了通过 CBZ6 诱导的氧化还原中性光催化制备的 β-氟烷基 α-羰基碳阳离子的亲核加成反应。在这个过程中,β-氟烷基α-羰基自由基的光催化氧化成相应的碳阳离子是关键步骤。β-氟烷基α-羰基自由基是通过向 α,β-不饱和羰基加成多氟烷基自由基而-羰基自由基在原位生成的。CBZ6 的高 E00 值(3.19 V 与饱和甘汞电极)对应于吸收的光能,有助于高催化反应性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号