Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational response of αIIbβ3 and αVβ3 integrins to force
Structure ( IF 4.4 ) Pub Date : 2024-12-19 , DOI: 10.1016/j.str.2024.11.016 Reza Kolasangiani, Khashayar Farzanian, Yunfeng Chen, Martin A. Schwartz, Tamara C. Bidone
Structure ( IF 4.4 ) Pub Date : 2024-12-19 , DOI: 10.1016/j.str.2024.11.016 Reza Kolasangiani, Khashayar Farzanian, Yunfeng Chen, Martin A. Schwartz, Tamara C. Bidone
|
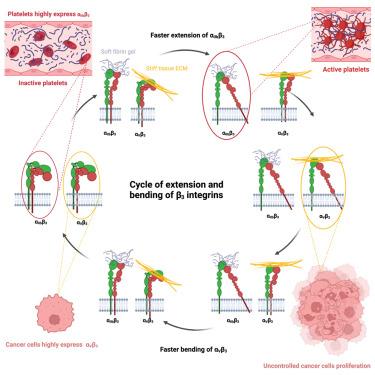
As major adhesion receptors, integrins transmit biochemical and mechanical signals across the plasma membrane. These functions are regulated by transitions between bent and extended conformations and modulated by force. To understand how force on integrins mediates cellular mechanosensing, we compared two highly homologous integrins, αIIbβ3 and αVβ3. These integrins, expressed in circulating platelets vs. solid tissues, respectively, share the β3 subunit, bind similar ligands and have similar bent and extended conformations. Here, we report that in cells expressing equivalent levels of each integrin, αIIbβ3 mediates spreading on softer substrates than αVβ3. These effects correlate with differences in structural dynamics of the two integrins under force. All-atom simulations show that αIIbβ3 is more flexible than αVβ3 due to correlated residue motions within the α subunit domains. Single molecule measurements confirm that αIIbβ3 extends faster than αVβ3. These results reveal a fundamental relationship between protein function and structural dynamics in cell mechanosensing.
中文翻译:

αIIbβ3 和 αVβ3 整合素对力的构象反应
作为主要的粘附受体,整合素跨质膜传递生化和机械信号。这些功能由弯曲和扩展构象之间的过渡调节,并受力调节。为了了解对整合素的力如何介导细胞机械感应,我们比较了两种高度同源的整合素,αIIbβ3 和 αVβ3。这些整合素分别在循环血小板和实体组织中表达,共享 β3 亚基,结合相似的配体,并具有相似的弯曲和延伸构象。在这里,我们报道了在表达等水平的每种整合素的细胞中,αIIbβ3 介导比α Vβ3 更软的底物上的扩散。这些效应与作用下两种整合素的结构动力学差异相关。全原子模拟表明,由于 α 亚基域内的相关残基运动,αIIbβ3 比 α βV3 更灵活。单分子测量证实,αIIbβ3 的延伸速度比 αVβ 3 快。这些结果揭示了细胞机械感应中蛋白质功能和结构动力学之间的基本关系。
更新日期:2024-12-19
中文翻译:

αIIbβ3 和 αVβ3 整合素对力的构象反应
作为主要的粘附受体,整合素跨质膜传递生化和机械信号。这些功能由弯曲和扩展构象之间的过渡调节,并受力调节。为了了解对整合素的力如何介导细胞机械感应,我们比较了两种高度同源的整合素,αIIbβ3 和 αVβ3。这些整合素分别在循环血小板和实体组织中表达,共享 β3 亚基,结合相似的配体,并具有相似的弯曲和延伸构象。在这里,我们报道了在表达等水平的每种整合素的细胞中,αIIbβ3 介导比α Vβ3 更软的底物上的扩散。这些效应与作用下两种整合素的结构动力学差异相关。全原子模拟表明,由于 α 亚基域内的相关残基运动,αIIbβ3 比 α βV3 更灵活。单分子测量证实,αIIbβ3 的延伸速度比 αVβ 3 快。这些结果揭示了细胞机械感应中蛋白质功能和结构动力学之间的基本关系。






























 京公网安备 11010802027423号
京公网安备 11010802027423号