Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic control of RNA-DNA hybrid formation orchestrates DNA2 activation at stalled forks by RNAPII and DDX39A
Molecular Cell ( IF 14.5 ) Pub Date : 2024-12-19 , DOI: 10.1016/j.molcel.2024.11.034 Lizhi Song, Haihua Xie, Haonan Fan, Yanjun Zhang, Zixiu Cheng, Junliang Chen, Yuzun Guo, Shudi Zhang, Xinyu Zhou, Zhaoshuang Li, Haoxiang Liao, Jinhua Han, Jun Huang, Jianwei Zhou, Dong Fang, Ting Liu
Molecular Cell ( IF 14.5 ) Pub Date : 2024-12-19 , DOI: 10.1016/j.molcel.2024.11.034 Lizhi Song, Haihua Xie, Haonan Fan, Yanjun Zhang, Zixiu Cheng, Junliang Chen, Yuzun Guo, Shudi Zhang, Xinyu Zhou, Zhaoshuang Li, Haoxiang Liao, Jinhua Han, Jun Huang, Jianwei Zhou, Dong Fang, Ting Liu
|
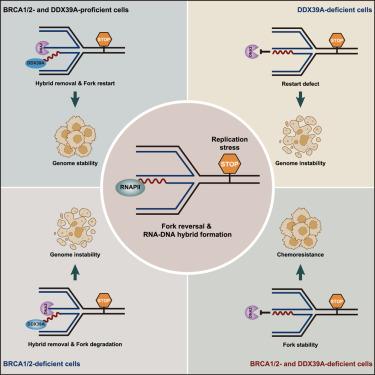
Stalled replication forks, susceptible to nucleolytic threats, necessitate protective mechanisms involving pivotal factors such as the tumor suppressors BRCA1 and BRCA2. Here, we demonstrate that, upon replication stress, RNA polymerase II (RNAPII) is recruited to stalled forks, actively promoting the transient formation of RNA-DNA hybrids. These hybrids act as safeguards, preventing premature engagement by the DNA2 nuclease and uncontrolled DNA2-mediated degradation of nascent DNA. Furthermore, we provide evidence that DExD box polypeptide 39A (DDX39A), serving as an RNA-DNA resolver, unwinds these structures and facilitates regulated DNA2 access to stalled forks. This orchestrated process enables controlled DNA2-dependent stalled fork processing and restart. Finally, we reveal that loss of DDX39A enhances stalled fork protection in BRCA1/2-deficient cells, consequently conferring chemoresistance. Our results suggest that the dynamic regulation of RNA-DNA hybrid formation at stalled forks by RNAPII and DDX39A precisely governs the timing of DNA2 activation, contributing to stalled fork protection, processing, and restart, ultimately promoting genome stability.
中文翻译:

RNA-DNA 杂交形成的动态控制通过 RNAPII 和 DDX39A 在停滞的分叉处协调 DNA2 激活
停滞的复制分叉容易受到溶核威胁,需要涉及关键因子(如肿瘤抑制因子 BRCA1 和 BRCA2)的保护机制。在这里,我们证明,在复制应激时,RNA 聚合酶 II (RNAPII) 被募集到停滞的叉子中,积极促进 RNA-DNA 杂交体的瞬时形成。这些杂交体起到保护作用,防止 DNA2 核酸酶过早结合和不受控制的 DNA2 介导的新生 DNA 降解。此外,我们提供的证据表明,DExD 盒多肽 39A (DDX39A) 作为 RNA-DNA 解析器,可以解开这些结构并促进受调节的 DNA2 进入停滞的叉子。这种精心编排的过程可实现受控的 DNA2 依赖性停滞叉处理和重新启动。最后,我们揭示了 DDX39A 的缺失增强了 BRCA1/2 缺陷细胞中的停滞叉保护,从而赋予化疗耐药性。我们的结果表明,RNAPII 和 DDX39A 对停滞分叉处 RNA-DNA 杂交形成的动态调节精确控制了 DNA2 激活的时间,有助于停滞分叉的保护、加工和重启,最终促进基因组稳定性。
更新日期:2024-12-19
中文翻译:

RNA-DNA 杂交形成的动态控制通过 RNAPII 和 DDX39A 在停滞的分叉处协调 DNA2 激活
停滞的复制分叉容易受到溶核威胁,需要涉及关键因子(如肿瘤抑制因子 BRCA1 和 BRCA2)的保护机制。在这里,我们证明,在复制应激时,RNA 聚合酶 II (RNAPII) 被募集到停滞的叉子中,积极促进 RNA-DNA 杂交体的瞬时形成。这些杂交体起到保护作用,防止 DNA2 核酸酶过早结合和不受控制的 DNA2 介导的新生 DNA 降解。此外,我们提供的证据表明,DExD 盒多肽 39A (DDX39A) 作为 RNA-DNA 解析器,可以解开这些结构并促进受调节的 DNA2 进入停滞的叉子。这种精心编排的过程可实现受控的 DNA2 依赖性停滞叉处理和重新启动。最后,我们揭示了 DDX39A 的缺失增强了 BRCA1/2 缺陷细胞中的停滞叉保护,从而赋予化疗耐药性。我们的结果表明,RNAPII 和 DDX39A 对停滞分叉处 RNA-DNA 杂交形成的动态调节精确控制了 DNA2 激活的时间,有助于停滞分叉的保护、加工和重启,最终促进基因组稳定性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号