Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DDX39A resolves replication fork-associated RNA-DNA hybrids to balance fork protection and cleavage for genomic stability maintenance
Molecular Cell ( IF 14.5 ) Pub Date : 2024-12-19 , DOI: 10.1016/j.molcel.2024.11.029 Zhanzhan Xu, Chen Nie, Junwei Liao, Yujie Ma, Xiao Albert Zhou, Xiaoman Li, Shiwei Li, Haodong Lin, Yefei Luo, Kaiqi Cheng, Zuchao Mao, Lei Zhang, Yichen Pan, Yuke Chen, Weibin Wang, Jiadong Wang
Molecular Cell ( IF 14.5 ) Pub Date : 2024-12-19 , DOI: 10.1016/j.molcel.2024.11.029 Zhanzhan Xu, Chen Nie, Junwei Liao, Yujie Ma, Xiao Albert Zhou, Xiaoman Li, Shiwei Li, Haodong Lin, Yefei Luo, Kaiqi Cheng, Zuchao Mao, Lei Zhang, Yichen Pan, Yuke Chen, Weibin Wang, Jiadong Wang
|
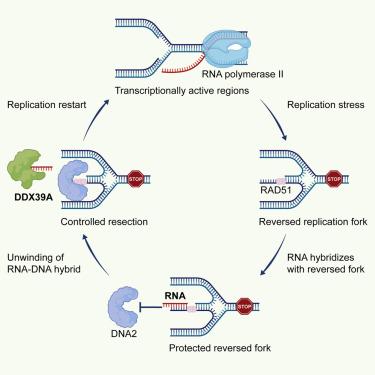
Safeguarding replication fork stability in transcriptionally active regions is crucial for precise DNA replication and mutation prevention. Here, we discover the pervasive existence of replication fork-associated RNA-DNA hybrids (RF-RDs) in transcriptionally active regions of human cells. These hybrids function as protective barriers, preventing DNA2-mediated nascent DNA degradation and replication fork collapse under replication stress. We also identify DDX39A as a RAD51-associated protein that binds to stalled forks and resolves RF-RDs, facilitating proper DNA2-mediated DNA resection and replication fork restart. Excessive dissolution of RF-RDs causes replication fork collapse and genomic instability, while insufficient dissolution of RF-RDs under replication stress increases fork stability, resulting in chemoresistance that can be reversed by eliminating RF-RDs. In summary, we elucidated the prevalence of RF-RDs at replication forks within transcriptionally active regions, revealed their pivotal role in safeguarding replication fork stability, and proposed that targeting RF-RDs holds promise for augmenting chemotherapeutic efficacy.
中文翻译:

DDX39A 解析复制叉相关的 RNA-DNA 杂交体,以平衡叉保护和切割,以维持基因组稳定性
保护转录活性区域的复制叉稳定性对于精确的 DNA 复制和突变预防至关重要。在这里,我们发现在人类细胞的转录活性区域普遍存在复制叉相关的 RNA-DNA 杂交体 (RF-RDs)。这些杂交体起到保护屏障的作用,防止 DNA2 介导的新生 DNA 降解和复制叉在复制应激下崩溃。我们还将 DDX39A 鉴定为 RAD51 相关蛋白,它与停滞的分叉结合并解析 RF-RD,促进 DNA2 介导的正确 DNA 切除和复制叉重启。RF-RDs 的过度溶解会导致复制叉崩溃和基因组不稳定,而 RF-RDs 在复制应力下的溶解不足会增加叉的稳定性,从而导致化疗耐药性,可以通过消除 RF-RDs 来逆转。总之,我们阐明了 RF-RDs 在转录活性区域内复制叉中的普遍性,揭示了它们在保护复制叉稳定性方面的关键作用,并提出靶向 RF-RDs 有望提高化疗疗效。
更新日期:2024-12-19
中文翻译:

DDX39A 解析复制叉相关的 RNA-DNA 杂交体,以平衡叉保护和切割,以维持基因组稳定性
保护转录活性区域的复制叉稳定性对于精确的 DNA 复制和突变预防至关重要。在这里,我们发现在人类细胞的转录活性区域普遍存在复制叉相关的 RNA-DNA 杂交体 (RF-RDs)。这些杂交体起到保护屏障的作用,防止 DNA2 介导的新生 DNA 降解和复制叉在复制应激下崩溃。我们还将 DDX39A 鉴定为 RAD51 相关蛋白,它与停滞的分叉结合并解析 RF-RD,促进 DNA2 介导的正确 DNA 切除和复制叉重启。RF-RDs 的过度溶解会导致复制叉崩溃和基因组不稳定,而 RF-RDs 在复制应力下的溶解不足会增加叉的稳定性,从而导致化疗耐药性,可以通过消除 RF-RDs 来逆转。总之,我们阐明了 RF-RDs 在转录活性区域内复制叉中的普遍性,揭示了它们在保护复制叉稳定性方面的关键作用,并提出靶向 RF-RDs 有望提高化疗疗效。






























 京公网安备 11010802027423号
京公网安备 11010802027423号