当前位置:
X-MOL 学术
›
Cell Host Microbe
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Roseburia intestinalis-derived butyrate alleviates neuropathic pain
Cell Host & Microbe ( IF 20.6 ) Pub Date : 2024-12-19 , DOI: 10.1016/j.chom.2024.11.013 Yanjun Jiang, Ziheng Huang, Wuping Sun, Jiabin Huang, Yunlong Xu, Yuliang Liao, Tingting Jin, Qing Li, Idy Hiu Ting Ho, Yidan Zou, Wenyi Zhu, Qian Li, Fenfen Qin, Xinyi Zhang, Shuqi Shi, Na Zhang, Shaomin Yang, Wenhui Xie, Songbin Wu, Likai Tan, Lin Zhang, Huarong Chen, Tony Gin, Matthew Tak Vai Chan, William Ka Kei Wu, Lizu Xiao, Xiaodong Liu
Cell Host & Microbe ( IF 20.6 ) Pub Date : 2024-12-19 , DOI: 10.1016/j.chom.2024.11.013 Yanjun Jiang, Ziheng Huang, Wuping Sun, Jiabin Huang, Yunlong Xu, Yuliang Liao, Tingting Jin, Qing Li, Idy Hiu Ting Ho, Yidan Zou, Wenyi Zhu, Qian Li, Fenfen Qin, Xinyi Zhang, Shuqi Shi, Na Zhang, Shaomin Yang, Wenhui Xie, Songbin Wu, Likai Tan, Lin Zhang, Huarong Chen, Tony Gin, Matthew Tak Vai Chan, William Ka Kei Wu, Lizu Xiao, Xiaodong Liu
|
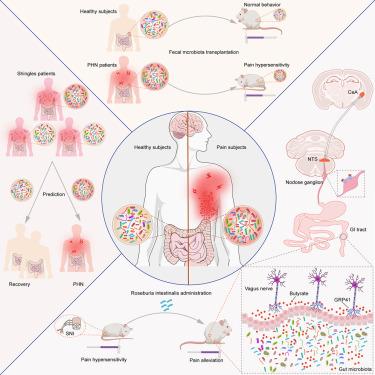
Approximately 20% of patients with shingles develop postherpetic neuralgia (PHN). We investigated the role of gut microbiota in shingle- and PHN-related pain. Patients with shingles or PHN exhibited significant alterations in their gut microbiota with microbial markers predicting PHN development among patients with shingles. Functionally, fecal microbiota transplantation from patients with PHN to mice heightened pain sensitivity. Administration of Roseburia intestinalis , a bacterium both depleted in patients with shingles and PHN, alleviated peripheral nerve injury-induced pain in mice. R. intestinalis enhanced vagal neurotransmission to the nucleus tractus solitarius (NTS) to suppress the central amygdala (CeA), a brain region involved in pain perception. R. intestinalis- generated butyrate activated vagal neurons through the receptor, G protein-coupled receptor 41 (GPR41). Vagal knockout of Gpr41 abolished the effects of R. intestinalis on the NTS-CeA circuit and reduced pain behaviors. Overall, we established a microbiota-based model for PHN risk assessment and identified R. intestinalis as a potential pain-alleviating probiotic.
中文翻译:

Roseburia 肠衍生的丁酸盐减轻神经性疼痛
大约 20% 的带状疱疹患者会发展为带状疱疹后神经痛 (PHN)。我们研究了肠道微生物群在带状疱疹和 PHN 相关疼痛中的作用。带状疱疹或 PHN 患者的肠道微生物群表现出显着变化,微生物标志物预测带状疱疹患者 PHN 的发展。从功能上讲,从 PHN 患者将粪便微生物群移植到小鼠中提高了疼痛敏感性。施用 Roseburia intestinalis(一种在带状疱疹和 PHN 患者中均已耗尽的细菌)减轻了小鼠周围神经损伤引起的疼痛。R. intestinalis 增强了迷走神经向孤束核 (NTS) 的传递,以抑制中枢杏仁核 (CeA),这是一个参与疼痛感知的大脑区域。R. intestalis 产生的丁酸盐通过受体 G 蛋白偶联受体 41 (GPR41) 激活迷走神经神经元。Gpr41 的迷走神经敲除消除了 R. intestinalis 对 NTS-CeA 回路的影响并减轻了疼痛行为。总体而言,我们建立了一个基于微生物群的 PHN 风险评估模型,并确定 R. intestinalis 是一种潜在的缓解疼痛的益生菌。
更新日期:2024-12-19
中文翻译:

Roseburia 肠衍生的丁酸盐减轻神经性疼痛
大约 20% 的带状疱疹患者会发展为带状疱疹后神经痛 (PHN)。我们研究了肠道微生物群在带状疱疹和 PHN 相关疼痛中的作用。带状疱疹或 PHN 患者的肠道微生物群表现出显着变化,微生物标志物预测带状疱疹患者 PHN 的发展。从功能上讲,从 PHN 患者将粪便微生物群移植到小鼠中提高了疼痛敏感性。施用 Roseburia intestinalis(一种在带状疱疹和 PHN 患者中均已耗尽的细菌)减轻了小鼠周围神经损伤引起的疼痛。R. intestinalis 增强了迷走神经向孤束核 (NTS) 的传递,以抑制中枢杏仁核 (CeA),这是一个参与疼痛感知的大脑区域。R. intestalis 产生的丁酸盐通过受体 G 蛋白偶联受体 41 (GPR41) 激活迷走神经神经元。Gpr41 的迷走神经敲除消除了 R. intestinalis 对 NTS-CeA 回路的影响并减轻了疼痛行为。总体而言,我们建立了一个基于微生物群的 PHN 风险评估模型,并确定 R. intestinalis 是一种潜在的缓解疼痛的益生菌。

































 京公网安备 11010802027423号
京公网安备 11010802027423号