当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced oxidation and in-situ coagulation Fe(Ⅱ)/peroxymonosulfate-Mn(Ⅶ) process for carbamazepine removal: Multiple promoting effects of Mn and direct/indirect regulation of Cl− on active substances transformation
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-18 , DOI: 10.1016/j.jhazmat.2024.136933 Yanli Kong, Siyu Dai, Qingwu Chen, Yong Nie, Jiangya Ma
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-18 , DOI: 10.1016/j.jhazmat.2024.136933 Yanli Kong, Siyu Dai, Qingwu Chen, Yong Nie, Jiangya Ma
|
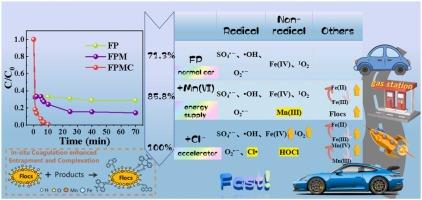
Slow transformation efficiency of Fe(III)/Fe(II) limits the generation of radicals in peroxymonosulfate (PMS)-based advanced oxidation processes (AOPs), and these radicals was easy to be interfered by the presence of water constituents. In addition, in-situ coagulation during this oxidation process was neglected. This study proposed Fe(II)/PMS-Mn(VII) in the presence of chlorides ions (FPMC) process to reveal multiple promoting effects of Mn on redox cycle of Fe(III)/Fe(II) and different reactive mechanisms of Cl− on types of radicals generation pathways, and the in-situ coagulation enhanced mechanisms was investigated. Results showed that the dual functionality of oxidation and in-situ coagulation in FPMC process was significantly enhanced that carbamazepine (CBZ) could be efficiently and quickly removed. The reduction product Mn(III) of Mn(VII) could promote the redox cycle of Mn(II)/Mn(III) and Mn(III)/Mn(IV) that facilitated Fe(III)/Fe(II), sustaining the reactivity of the system. Cl− could significantly promote the cycling of Mn(Ⅲ)/Mn(Ⅳ) that indirectly affected the cycle of Fe(III)/Fe(II).
中文翻译:

增强氧化和原位凝固 Fe(II.)/过氧一硫酸盐-Mn(VII.) 去除卡马西平的过程:Mn 和 Cl− 的直接/间接调节对活性物质转化的多重促进作用
Fe(III)/Fe(II) 的缓慢转化效率限制了基于过氧一硫酸盐 (PMS) 的高级氧化过程 (AOP) 中自由基的产生,并且这些自由基很容易受到水成分存在的干扰。此外,该氧化过程中的原位凝结被忽略了。本研究提出在氯离子 (FPMC) 存在下使用 Fe(II)/PMS-Mn(VII) 工艺,揭示 Mn 对 Fe(III)/Fe(II) 氧化还原循环的多重促进作用以及 Cl − 对自由基生成途径类型的不同反应机制,并研究了原位凝血增强机制。结果表明,FPMC 过程中氧化和原位凝血的双重功能显著增强,可以高效、快速地去除卡马西平 (CBZ)。Mn(VII) 的还原产物 Mn(III) 可以促进 Mn(II)/Mn(III) 和 Mn(III)/Mn(IV) 的氧化还原循环,从而促进 Fe(III)/Fe(II),维持体系的反应性。Cl − 可显著促进 Mn(III.)/Mn(IV.) 的循环,间接影响 Fe(III)/Fe(II) 的循环。
更新日期:2024-12-20
中文翻译:

增强氧化和原位凝固 Fe(II.)/过氧一硫酸盐-Mn(VII.) 去除卡马西平的过程:Mn 和 Cl− 的直接/间接调节对活性物质转化的多重促进作用
Fe(III)/Fe(II) 的缓慢转化效率限制了基于过氧一硫酸盐 (PMS) 的高级氧化过程 (AOP) 中自由基的产生,并且这些自由基很容易受到水成分存在的干扰。此外,该氧化过程中的原位凝结被忽略了。本研究提出在氯离子 (FPMC) 存在下使用 Fe(II)/PMS-Mn(VII) 工艺,揭示 Mn 对 Fe(III)/Fe(II) 氧化还原循环的多重促进作用以及 Cl − 对自由基生成途径类型的不同反应机制,并研究了原位凝血增强机制。结果表明,FPMC 过程中氧化和原位凝血的双重功能显著增强,可以高效、快速地去除卡马西平 (CBZ)。Mn(VII) 的还原产物 Mn(III) 可以促进 Mn(II)/Mn(III) 和 Mn(III)/Mn(IV) 的氧化还原循环,从而促进 Fe(III)/Fe(II),维持体系的反应性。Cl − 可显著促进 Mn(III.)/Mn(IV.) 的循环,间接影响 Fe(III)/Fe(II) 的循环。






























 京公网安备 11010802027423号
京公网安备 11010802027423号