Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Constructing Lewis Acid–Base Pairs to Boost Electrocatalytic Hydrogenation of p‐Nitrobenzoic Acid to Valuable p‐Aminobenzoic Acid Using Water as the Hydrogen Source
Small ( IF 13.0 ) Pub Date : 2024-12-19 , DOI: 10.1002/smll.202409455 Gang Xu, Shi‐Jiao Dong, Han‐Jian Liu, Wen‐Biao Wang, Xi‐Min Zhang, Mei‐Qing Cai, Jiansong Sun, Jun‐Ling Song
Small ( IF 13.0 ) Pub Date : 2024-12-19 , DOI: 10.1002/smll.202409455 Gang Xu, Shi‐Jiao Dong, Han‐Jian Liu, Wen‐Biao Wang, Xi‐Min Zhang, Mei‐Qing Cai, Jiansong Sun, Jun‐Ling Song
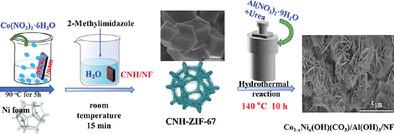
|
Electrocatalytic hydrogenation of toxic nitrobenzene to value‐added aniline is of great significance in addressing the issues of energy crisis and environmental pollution. However, it is a considerable challenging and crucial to develop highly efficient and earth‐abundant transition metal‐based electrocatalysts with superior durability for the electro‐hydrogenation of nitrobenzene due to the competitive hydrogen evolution reaction (HER). In this work, a facile approach is designed and introduced to constructing an integrated self‐supported heterostructured Co1‐ x x 3 )/Al(OH)3 nanoarrays (CoNiCH/Al(OH)3 ) for the electrocatalytic reduction of nitrobenzoic acid (PNBA) to p‐aminobenzoic acid (PABA) and its electrocatalytic mechanism for PNBA reduction is investigated. This unique Lewis acid–base pairs with abundant oxygen vacancies (OVs) can effectively regulate the adsorption energy of PNBA and active hydrogen intermediates, facilitate the proton‐coupled electrocatalytic reduction process, leading to the high activity and selectivity for PNBA to PABA. The optimal CoNiCH/Al(OH)3 ‐0.5 exhibits outstanding performance for the electrocatalytic hydrogenation of PNBA to PABA with a yield of 92%, selectivity of 95% and Faraday efficiency (FE) of 92% at ‐0.545 V (vs reversible hydrogen electrode, RHE) under 0.1 m phosphate buffered solution (PBS) neutral electrolyte. Besides, it can maintain a high electrocatalytic activity for at least eight electrocatalytic cycle‐test.
中文翻译:

构建路易斯酸-碱对,以水为氢源,促进对硝基苯甲酸电催化加氢生成有价值的对氨基苯甲酸
有毒硝基苯电催化加氢制高附加值苯胺对解决能源危机和环境污染问题具有重要意义。然而,由于竞争性析氢反应 (HER),开发高效且地球上丰富的过渡金属基电催化剂对于硝基苯的电加氢反应具有卓越的耐久性,这是一个相当大的挑战和关键。在这项工作中,设计并介绍了一种简单的方法来构建一种集成的自支撑异质结构 Co 1- x Ni x (OH)(CO 3 )/Al(OH) 3 纳米阵列 (CoNiCH/Al(OH) 3 ) 用于硝基苯甲酸 (PNBA) 电催化还原对氨基苯甲酸 (PABA) 及其电催化还原 PNBA 的机制。这种独特的路易斯酸碱对具有丰富的氧空位 (OVs) 可以有效调节 PNBA 和活性氢中间体的吸附能,促进质子耦合电催化还原过程,导致 PNBA 到 PABA 的高活性和选择性。最佳 CoNiCH/Al(OH) 3 -0.5 在 -0.545 V (与可逆氢电极, RHE] 相比) 下在 0.1 m 磷酸盐缓冲溶液 (PBS) 中性电解质下表现出优异的 PNBA 电催化加氢制 PABA,产率为 92%,选择性为 95%,法拉第效率 (FE) 为 92%。此外,它可以在至少 8 次电催化循环测试中保持较高的电催化活性。
更新日期:2024-12-19
中文翻译:

构建路易斯酸-碱对,以水为氢源,促进对硝基苯甲酸电催化加氢生成有价值的对氨基苯甲酸
有毒硝基苯电催化加氢制高附加值苯胺对解决能源危机和环境污染问题具有重要意义。然而,由于竞争性析氢反应 (HER),开发高效且地球上丰富的过渡金属基电催化剂对于硝基苯的电加氢反应具有卓越的耐久性,这是一个相当大的挑战和关键。在这项工作中,设计并介绍了一种简单的方法来构建一种集成的自支撑异质结构 Co 1- x Ni x (OH)(CO 3 )/Al(OH) 3 纳米阵列 (CoNiCH/Al(OH) 3 ) 用于硝基苯甲酸 (PNBA) 电催化还原对氨基苯甲酸 (PABA) 及其电催化还原 PNBA 的机制。这种独特的路易斯酸碱对具有丰富的氧空位 (OVs) 可以有效调节 PNBA 和活性氢中间体的吸附能,促进质子耦合电催化还原过程,导致 PNBA 到 PABA 的高活性和选择性。最佳 CoNiCH/Al(OH) 3 -0.5 在 -0.545 V (与可逆氢电极, RHE] 相比) 下在 0.1 m 磷酸盐缓冲溶液 (PBS) 中性电解质下表现出优异的 PNBA 电催化加氢制 PABA,产率为 92%,选择性为 95%,法拉第效率 (FE) 为 92%。此外,它可以在至少 8 次电催化循环测试中保持较高的电催化活性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号