当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting ATM enhances radiation sensitivity of colorectal cancer by Potentiating radiation-induced cell death and antitumor immunity
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-12-19 , DOI: 10.1016/j.jare.2024.12.023 Yuwen Xie, Yang Liu, Mingdao Lin, Zhenkang Li, Zhiyong Shen, Shengqi Yin, Yilin Zheng, Yishu Zou, Yaowei Zhang, Yizhi Zhan, Yuan Fang, Yi Ding
中文翻译:

靶向 ATM 通过增强辐射诱导的细胞死亡和抗肿瘤免疫来提高结直肠癌的辐射敏感性
结直肠癌 (CRC) 的放疗疗效通常受到辐射耐药性的限制。共济失调毛细血管扩张症突变 (ATM) 因其在修复 DNA 损伤反应 (DDR) 通路中的双链 DNA 断裂中的作用而广为人知。然而,ATM 是否介导导致辐射抗性的其他机制仍未得到充分研究。
本研究调查了靶向 ATM 如何提高 CRC 辐射敏感性,并评估联合策略以改善放射治疗结果。
分析临床标本以将 ATM 激活与放疗反应相关联。功能测定,包括 EdU 、细胞活力、克隆形成存活和细胞凋亡测定,用于评估 ATM 抑制对辐射敏感性的影响。通过 RNA-seq 、 RT-qPCR 、 western blotting、 ELISA 、 免疫荧光 、 流式细胞术 、 ChIP-qPCR 和免疫共沉淀获得机制见解。使用皮下肿瘤模型在裸鼠、BALB/c 和 C57BL/6J 小鼠中评价体内疗效。
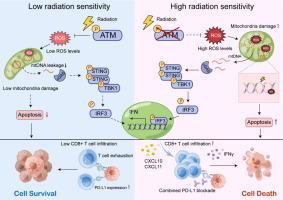
高 ATM 磷酸化水平与 CRC 患者的放疗反应不良相关。ATM 抑制增强了体外和体内模型的辐射敏感性。从机制上讲,ATM 抑制增加了辐射诱导的 ROS 积累和线粒体损伤,导致线粒体 DNA (mtDNA) 释放到胞质溶胶中并激活 STING I 型干扰素通路。这增强了 CD8+ T 细胞浸润并增强了抗肿瘤免疫力。此外,ATM 抑制部分减轻了辐射诱导的 PD-L1 上调,可能是通过 ATM/NEMO/NF-κB 通路。值得注意的是,放疗、ATM 抑制剂和抗 PD-L1 联合疗法在小鼠模型(包括大型、难治性肿瘤)中实现了卓越的肿瘤控制和缓解。
靶向 ATM 可增强辐射诱导的肿瘤细胞死亡并增强抗肿瘤免疫反应,为克服 CRC 辐射耐药提供了一种有前途的策略。放疗、ATM 抑制和免疫检查点阻断的协同作用突出了 CRC 管理的新治疗方法。
更新日期:2024-12-19
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-12-19 , DOI: 10.1016/j.jare.2024.12.023 Yuwen Xie, Yang Liu, Mingdao Lin, Zhenkang Li, Zhiyong Shen, Shengqi Yin, Yilin Zheng, Yishu Zou, Yaowei Zhang, Yizhi Zhan, Yuan Fang, Yi Ding
|
Introduction
The efficacy of radiotherapy in colorectal cancer (CRC) is often limited by radiation resistance. Ataxia telangiectasia mutated (ATM) is well known for its role in repairing double-strand DNA breaks within the DNA damage response (DDR) pathway. However, whether ATM mediates other mechanisms contributing to radiation resistance remains insufficiently investigated.Objectives
This study investigates how targeting ATM enhances CRC radiation sensitivity and evaluates combination strategies to improve radiotherapy outcomes.Methods
Clinical specimens were analyzed to correlate ATM activation with radiotherapy response. Functional assays, including EdU, cell viability, clonogenic survival, and apoptosis assays, were used to assess the impact of ATM inhibition on radiation sensitivity. Mechanistic insights were gained through RNA-seq, RT-qPCR, western blotting, ELISA, immunofluorescence, flow cytometry, ChIP-qPCR, and co-immunoprecipitation. In vivo efficacy was evaluated using subcutaneous tumor models in nude, BALB/c, and C57BL/6J mice.Results
High ATM phosphorylation levels correlated with poor radiotherapy response in CRC patients. ATM inhibition enhanced radiation sensitivity in both in vitro and in vivo models. Mechanistically, ATM inhibition increased radiation-induced ROS accumulation and mitochondrial damage, leading to the release of mitochondrial DNA (mtDNA) into the cytosol and activation of the STING-type I interferon pathway. This enhanced CD8+ T cell infiltration and boosted antitumor immunity. Additionally, ATM inhibition partially alleviated the radiation-induced upregulation of PD-L1, likely through the ATM/NEMO/NF-κB pathway. Notably, triple therapy combining radiotherapy, an ATM inhibitor, and anti-PD-L1 achieved superior tumor control and remission in mouse models, including large, treatment-resistant tumors.Conclusion
Targeting ATM enhances radiation-induced tumor cell death and boosts antitumor immune responses, offering a promising strategy to overcome CRC radiation resistance. The synergy of radiotherapy, ATM inhibitior, and immune checkpoint blockade highlights a novel therapeutic approach for CRC management.中文翻译:

靶向 ATM 通过增强辐射诱导的细胞死亡和抗肿瘤免疫来提高结直肠癌的辐射敏感性
介绍
结直肠癌 (CRC) 的放疗疗效通常受到辐射耐药性的限制。共济失调毛细血管扩张症突变 (ATM) 因其在修复 DNA 损伤反应 (DDR) 通路中的双链 DNA 断裂中的作用而广为人知。然而,ATM 是否介导导致辐射抗性的其他机制仍未得到充分研究。
目标
本研究调查了靶向 ATM 如何提高 CRC 辐射敏感性,并评估联合策略以改善放射治疗结果。
方法
分析临床标本以将 ATM 激活与放疗反应相关联。功能测定,包括 EdU 、细胞活力、克隆形成存活和细胞凋亡测定,用于评估 ATM 抑制对辐射敏感性的影响。通过 RNA-seq 、 RT-qPCR 、 western blotting、 ELISA 、 免疫荧光 、 流式细胞术 、 ChIP-qPCR 和免疫共沉淀获得机制见解。使用皮下肿瘤模型在裸鼠、BALB/c 和 C57BL/6J 小鼠中评价体内疗效。
结果
高 ATM 磷酸化水平与 CRC 患者的放疗反应不良相关。ATM 抑制增强了体外和体内模型的辐射敏感性。从机制上讲,ATM 抑制增加了辐射诱导的 ROS 积累和线粒体损伤,导致线粒体 DNA (mtDNA) 释放到胞质溶胶中并激活 STING I 型干扰素通路。这增强了 CD8+ T 细胞浸润并增强了抗肿瘤免疫力。此外,ATM 抑制部分减轻了辐射诱导的 PD-L1 上调,可能是通过 ATM/NEMO/NF-κB 通路。值得注意的是,放疗、ATM 抑制剂和抗 PD-L1 联合疗法在小鼠模型(包括大型、难治性肿瘤)中实现了卓越的肿瘤控制和缓解。
结论
靶向 ATM 可增强辐射诱导的肿瘤细胞死亡并增强抗肿瘤免疫反应,为克服 CRC 辐射耐药提供了一种有前途的策略。放疗、ATM 抑制和免疫检查点阻断的协同作用突出了 CRC 管理的新治疗方法。






























 京公网安备 11010802027423号
京公网安备 11010802027423号