当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
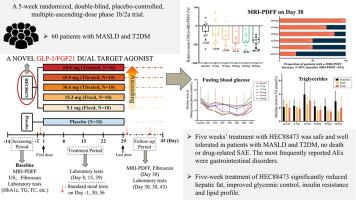
Safety and efficacy of GLP-1/FGF21 dual agonist HEC88473 in MASLD and T2DM: a randomized, double-blind, placebo-controlled study
Journal of Hepatology ( IF 26.8 ) Pub Date : 2024-12-19 , DOI: 10.1016/j.jhep.2024.12.006 Lin Xiang, Guixia Wang, Yulei Zhuang, Lin Luo, Jiangyu Yan, Hong Zhang, Xiaojiao Li, Can Xie, Qingwei He, Yuyu Peng, Hong Chen, Qianqian Li, Xiaoping Li, Linfeng Guo, Guoyue Lv, Yanhua Ding
更新日期:2024-12-19
Journal of Hepatology ( IF 26.8 ) Pub Date : 2024-12-19 , DOI: 10.1016/j.jhep.2024.12.006 Lin Xiang, Guixia Wang, Yulei Zhuang, Lin Luo, Jiangyu Yan, Hong Zhang, Xiaojiao Li, Can Xie, Qingwei He, Yuyu Peng, Hong Chen, Qianqian Li, Xiaoping Li, Linfeng Guo, Guoyue Lv, Yanhua Ding
|






























 京公网安备 11010802027423号
京公网安备 11010802027423号