当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective Copolymerization of Glucose-Derived Allopyranoside Epoxide with Cyclic Anhydrides: Developing Precise Sugar-Functionalized Polyesters with Unique Altrose Linkages
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-19 , DOI: 10.1021/jacs.4c13984 Jiaxi Xu, Jingjing Liu, Nikos Hadjichristidis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-19 , DOI: 10.1021/jacs.4c13984 Jiaxi Xu, Jingjing Liu, Nikos Hadjichristidis
|
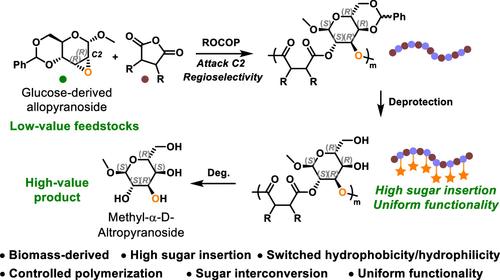
Uniform sugar-functionalized polyesters combine the benefits of sugar’s structural diversity, biocompatibility, and biodegradability with precise postfunctionalization capabilities, making them a highly valuable class of materials with extensive application potential. However, the irregular placement of hydroxyl groups has limited the synthesis of these polyesters. Here, we present the first platform for uniform sugar-functionalized polyesters via regioselective ring-opening copolymerizations (ROCOPs) of allopyranoside anhydrosugar epoxide (1, derived from d-glucose) with cyclic anhydrides, followed by complete selective deprotection. This method yields polyesters with controlled molecular weights, narrow molecular weight distributions (D̵ < 1.19), high glass transition temperatures (up to 188 °C), and uniform hydroxyl functionality. Furthermore, the degradation of the polyesters offers an efficient route for producing the highly valuable d-altrose. Mechanistic insights, supported by DFT calculations, as well as NMR and HPLC analyses, confirm the regioselective nucleophilic attack at the C2 position of the pyranose ring. Kinetic studies reveal a first-order dependence on 1 and a zero-order dependence on the cyclic anhydrides. Additionally, these uniform sugar-functionalized polyesters can be incorporated into triblock terpolymers through one-pot/one-step or one-pot/two-step procedures, forming uniform sugar-functionalized multiblock copolymers.
中文翻译:

葡萄糖衍生的异吡喃糖苷环氧化物与环状酸酐的区域选择性共聚:开发具有独特酪糖键的精确糖官能化聚酯
均匀的糖官能化聚酯将糖的结构多样性、生物相容性和生物降解性等优点与精确的后功能化能力相结合,使其成为一类具有广泛应用潜力的非常有价值的材料。然而,羟基的不规则放置限制了这些聚酯的合成。在这里,我们提出了第一个通过别吡喃糖苷脱水糖环氧化物(1,衍生自 d-葡萄糖)的区域选择性开环共聚 (ROCOP) 与环状酸酐实现均匀糖官能化聚酯的平台,然后完全选择性脱保护。该方法产生的聚酯具有可控的分子量、窄分子量分布 (D̵ < 1.19)、高玻璃化转变温度(高达 188 °C)和均匀的羟基官能团。此外,聚酯的降解为生产高价值的 d-烯丙糖提供了一种有效的途径。在 DFT 计算以及 NMR 和 HPLC 分析的支持下,机理见解证实了吡喃糖环 C2 位置的区域选择性亲核攻击。动力学研究揭示了对 1 的一阶依赖性和对环状酐的零级依赖性。此外,这些均匀的糖官能化聚酯可以通过一锅/一步法或一锅法/两步法掺入三嵌段三元共聚物中,形成均匀的糖官能化多嵌段共聚物。
更新日期:2024-12-19
中文翻译:

葡萄糖衍生的异吡喃糖苷环氧化物与环状酸酐的区域选择性共聚:开发具有独特酪糖键的精确糖官能化聚酯
均匀的糖官能化聚酯将糖的结构多样性、生物相容性和生物降解性等优点与精确的后功能化能力相结合,使其成为一类具有广泛应用潜力的非常有价值的材料。然而,羟基的不规则放置限制了这些聚酯的合成。在这里,我们提出了第一个通过别吡喃糖苷脱水糖环氧化物(1,衍生自 d-葡萄糖)的区域选择性开环共聚 (ROCOP) 与环状酸酐实现均匀糖官能化聚酯的平台,然后完全选择性脱保护。该方法产生的聚酯具有可控的分子量、窄分子量分布 (D̵ < 1.19)、高玻璃化转变温度(高达 188 °C)和均匀的羟基官能团。此外,聚酯的降解为生产高价值的 d-烯丙糖提供了一种有效的途径。在 DFT 计算以及 NMR 和 HPLC 分析的支持下,机理见解证实了吡喃糖环 C2 位置的区域选择性亲核攻击。动力学研究揭示了对 1 的一阶依赖性和对环状酐的零级依赖性。此外,这些均匀的糖官能化聚酯可以通过一锅/一步法或一锅法/两步法掺入三嵌段三元共聚物中,形成均匀的糖官能化多嵌段共聚物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号