当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
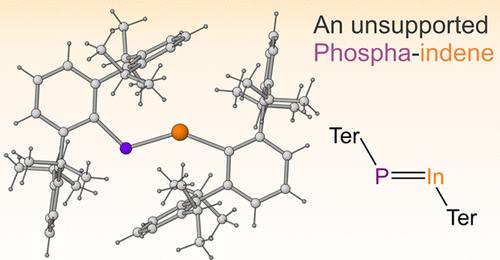
A Crystalline Unsupported Phosphagallene and Phosphaindene
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-19 , DOI: 10.1021/jacs.4c15041 Álvaro García-Romero, Chenyang Hu, Maren Pink, Jose M. Goicoechea
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-19 , DOI: 10.1021/jacs.4c15041 Álvaro García-Romero, Chenyang Hu, Maren Pink, Jose M. Goicoechea
|
The synthesis and isolation of TerP═GaTer and TerP═InTer (Ter = 2,6-Dipp2-C6H3; Dipp = 2,6-diisopropylphenyl) is reported. These compounds feature unsupported P═Ga and P═In double bonds and two-coordinate triel element centers. Key to the stabilization of such compounds is the steric bulk of the terphenyl substituents, which serve to shield the highly reactive P═E bonds (E = Ga, In) and prevent further aggregation. When smaller aromatic substituents are employed on the phosphorus-containing precursor, the cyclic compounds Mes*P(ETer)2 (Mes* = 2,4,6-tBu3-C6H2) are isolated instead. These species contain weakly aromatic three-membered rings. The presence of an external base (PMe3) is required in order to stabilize a phosphagallene when the smaller Mes* substituent is used, allowing for the isolation of Mes*P═GaTer(PMe3).
中文翻译:

A 结晶无支撑 Phosphagallene 和 Phosphaindene
TerP═GaTer 和 TerP═InTer 的合成和分离 (Ter = 2,6-DipP2-C 6H3;Dipp = 2,6-二异丙基苯基)的报道。这些化合物具有不支持的 P═Ga 和 P═In 双键以及双配位三重元素中心。稳定此类化合物的关键是三联苯取代基的空间本体,它用于保护高反应性 P═E 键 (E = Ga, In) 并防止进一步聚集。当在含磷前驱体上使用较小的芳香族取代基时,环状化合物 Mes*P(ETer)2 (Mes* = 2,4,6-t Bu3-C 6H2) 被分离出来。这些物种包含弱芳香族三元环。当使用较小的 Mes* 取代基时,为了稳定磷酸没食子烯,需要存在外部碱基 (PMe3),从而允许分离 Mes*P═GaTer (PMe3)。
更新日期:2024-12-19
中文翻译:

A 结晶无支撑 Phosphagallene 和 Phosphaindene
TerP═GaTer 和 TerP═InTer 的合成和分离 (Ter = 2,6-DipP2-C 6H3;Dipp = 2,6-二异丙基苯基)的报道。这些化合物具有不支持的 P═Ga 和 P═In 双键以及双配位三重元素中心。稳定此类化合物的关键是三联苯取代基的空间本体,它用于保护高反应性 P═E 键 (E = Ga, In) 并防止进一步聚集。当在含磷前驱体上使用较小的芳香族取代基时,环状化合物 Mes*P(ETer)2 (Mes* = 2,4,6-t Bu3-C 6H2) 被分离出来。这些物种包含弱芳香族三元环。当使用较小的 Mes* 取代基时,为了稳定磷酸没食子烯,需要存在外部碱基 (PMe3),从而允许分离 Mes*P═GaTer (PMe3)。






























 京公网安备 11010802027423号
京公网安备 11010802027423号