当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Carbonylative Cyclization of Alkenes with C–H Bonds for Synthesis of γ-Lactams Bearing an α-Quaternary Carbon
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-19 , DOI: 10.1021/jacs.4c15875 Weiwei Xu, Yanan Sun, Yuqing Jiang, Xueyuan Yan, Zhixuan Gao, Haorui Wang, Genping Huang, Qi-Lin Zhou, Mengchun Ye
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-19 , DOI: 10.1021/jacs.4c15875 Weiwei Xu, Yanan Sun, Yuqing Jiang, Xueyuan Yan, Zhixuan Gao, Haorui Wang, Genping Huang, Qi-Lin Zhou, Mengchun Ye
|
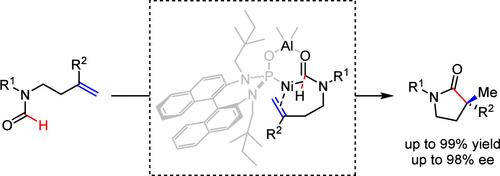
The development of effective synthetic methods to construct γ-lactams bearing a chiral α-quaternary carbon from relatively inert C(O)–H bonds with alkenes has been an elusive challenge. Herein, we used a naphthylamine-derived phosphine oxide ligating Ni and Al bimetallic catalyst to realize a carbonylative cyclization of formyl C–H bonds with alkenes, highly regio- and enantioselectively constructing γ-lactams bearing a chiral α-quaternary carbon in up to 99% yield and 98% ee. These γ-lactams proved to be versatile synthetic precursors for many biologically active molecules.
中文翻译:

烯烃与 C-H 键的对映选择性羰基环化反应,用于合成带有 α-季碳的 γ-内酰胺
从与烯烃相对惰性的 C(O)-H 键构建带有手性 α-季碳的 γ-内酰胺的有效合成方法一直是一项难以捉摸的挑战。在此,我们使用萘胺衍生的氧化膦连接 Ni 和 Al 双金属催化剂实现甲酰 C-H 键与烯烃的羰基环化,高度区域和对映选择性构建带有手性 α-季碳的 γ-内酰胺,产率高达 99% 和 98% ee。这些 γ-内酰胺类被证明是许多生物活性分子的多功能合成前体。
更新日期:2024-12-19
中文翻译:

烯烃与 C-H 键的对映选择性羰基环化反应,用于合成带有 α-季碳的 γ-内酰胺
从与烯烃相对惰性的 C(O)-H 键构建带有手性 α-季碳的 γ-内酰胺的有效合成方法一直是一项难以捉摸的挑战。在此,我们使用萘胺衍生的氧化膦连接 Ni 和 Al 双金属催化剂实现甲酰 C-H 键与烯烃的羰基环化,高度区域和对映选择性构建带有手性 α-季碳的 γ-内酰胺,产率高达 99% 和 98% ee。这些 γ-内酰胺类被证明是许多生物活性分子的多功能合成前体。






























 京公网安备 11010802027423号
京公网安备 11010802027423号