当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Indirect Formation of Peptide Bonds as a Prelude to Ribosomal Transpeptidation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-18 , DOI: 10.1021/jacs.4c10326 Harvey J. A. Dale, John D. Sutherland
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-18 , DOI: 10.1021/jacs.4c10326 Harvey J. A. Dale, John D. Sutherland
|
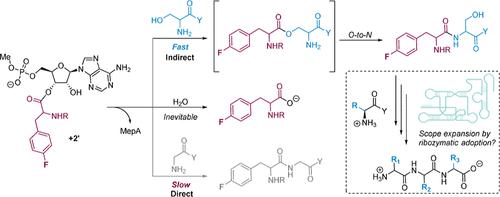
The catalytic competency of the ribosome in extant protein biosynthesis is thought to arise primarily from two sources: an ability to precisely juxtapose the termini of two key substrates─3′-aminoacyl and N-acyl-aminoacyl tRNAs─and an ability to ease direct transpeptidation by their desolvation and encapsulation. In the absence of ribosomal, or enzymatic, protection, however, these activated alkyl esters undergo efficient hydrolysis, while significant entropic barriers serve to hamper their intermolecular cross-aminolysis in bulk water. Given that the spontaneous emergence of a catalyst of comparable size and sophistication to the ribosome in a prebiotic RNA world would appear implausible, it is thus natural to ask how appreciable peptide formation could have occurred with such substrates in bulk water without the aid of advanced ribozymatic catalysis. Using a combination of fluorine-tagged aminoacyl adenylate esters, in situ monitoring by 19F{1H} NMR spectroscopy, analytical deconvolution of kinetics, pH–rate profile analysis, and temperature-dependence studies, we here explore the mechanistic landscape of indirect amidation, via transesterification and O-to-N rearrangement, as a highly efficient, alternative manifold for transpeptidation that may have served as a prelude to ribosomal peptide synthesis. Our results suggest a potentially overlooked role for those amino acids implicated by the cyanosulfidic reaction network with hydroxyl side chains (Ser and Thr), and they also help to resolve some outstanding ambiguities in the broader literature regarding studies of similar systems (e.g., aminolyzes with Tris buffer). The evolutionary implications of this mode of peptide synthesis and the involvement of a very specific subset of amino acids are discussed.
中文翻译:

间接形成肽键作为核糖体转肽化的前奏
核糖体在现存蛋白质生物合成中的催化能力被认为主要来自两个来源:精确并列两种关键底物(3′-氨酰基和 N-酰基-氨酰基 tRNA)末端的能力,以及通过它们的去溶剂化和封装来缓解直接转肽的能力。然而,在没有核糖体或酶保护的情况下,这些活化的烷基酯会进行有效的水解,而显着的熵垒会阻碍它们在散装水中进行分子间交叉氨基分解。鉴于在益生元 RNA 世界中自发出现与核糖体大小和复杂程度相当的催化剂似乎是不可信的,因此很自然地会问,如果没有先进的核糖催化的帮助,这种底物在散装水中是如何发生明显的肽形成的。使用氟标记的氨酰腺苷酸酯、通过 19F{1H} NMR 波谱进行原位监测、动力学分析反卷积、pH 速率分布分析和温度依赖性研究的组合,我们在这里探讨了通过酯交换和 O-to-N 重排间接酰胺化的机理景观,作为一种高效的转肽替代歧管,可能作为核糖体肽合成的前奏。我们的结果表明,那些与羟基侧链 (Ser 和 Thr) 的氰硫反应网络相关的氨基酸可能被忽视的作用,它们还有助于解决更广泛文献中关于类似系统研究的一些突出的歧义(例如,用 Tris 缓冲液进行氨基溶解)。 讨论了这种肽合成模式的进化意义和非常特定的氨基酸子集的参与。
更新日期:2024-12-18
中文翻译:

间接形成肽键作为核糖体转肽化的前奏
核糖体在现存蛋白质生物合成中的催化能力被认为主要来自两个来源:精确并列两种关键底物(3′-氨酰基和 N-酰基-氨酰基 tRNA)末端的能力,以及通过它们的去溶剂化和封装来缓解直接转肽的能力。然而,在没有核糖体或酶保护的情况下,这些活化的烷基酯会进行有效的水解,而显着的熵垒会阻碍它们在散装水中进行分子间交叉氨基分解。鉴于在益生元 RNA 世界中自发出现与核糖体大小和复杂程度相当的催化剂似乎是不可信的,因此很自然地会问,如果没有先进的核糖催化的帮助,这种底物在散装水中是如何发生明显的肽形成的。使用氟标记的氨酰腺苷酸酯、通过 19F{1H} NMR 波谱进行原位监测、动力学分析反卷积、pH 速率分布分析和温度依赖性研究的组合,我们在这里探讨了通过酯交换和 O-to-N 重排间接酰胺化的机理景观,作为一种高效的转肽替代歧管,可能作为核糖体肽合成的前奏。我们的结果表明,那些与羟基侧链 (Ser 和 Thr) 的氰硫反应网络相关的氨基酸可能被忽视的作用,它们还有助于解决更广泛文献中关于类似系统研究的一些突出的歧义(例如,用 Tris 缓冲液进行氨基溶解)。 讨论了这种肽合成模式的进化意义和非常特定的氨基酸子集的参与。

































 京公网安备 11010802027423号
京公网安备 11010802027423号