当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective α-C(sp3)–H Borylation of Masked Primary Alcohols Enabled by Iridium Catalysis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-18 , DOI: 10.1021/jacs.4c14890 Qian Gao, Yinwu Li, Lili Chen, Liang-Jun Xie, Xiangfeng Shao, Zhuofeng Ke, Senmiao Xu
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-18 , DOI: 10.1021/jacs.4c14890 Qian Gao, Yinwu Li, Lili Chen, Liang-Jun Xie, Xiangfeng Shao, Zhuofeng Ke, Senmiao Xu
|
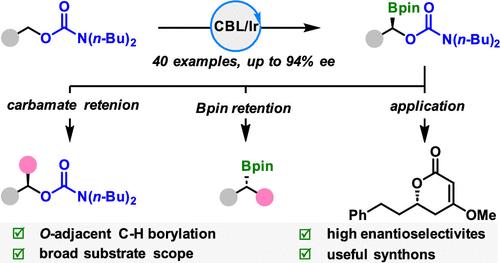
Functional group-directed site- and enantioselective C(sp3)–H functionalization of alcohols or masked alcohols represents a formidable challenge. We herein report the first example of iridium-catalyzed asymmetric α-C(sp3)–H borylation of primary alcohol-derived carbamates by the judicious choice of directing groups. A variety of chiral borylated carbamates were obtained with good to high enantioselectivities. We also demonstrated the synthetic utility by taking advantage of the highly transformable feature of C–B bonds and the leaving ability of carbamates.
中文翻译:

铱催化实现掩蔽伯醇的对映选择性 α-C(sp3)–H 硼酸化
醇或掩蔽醇的官能团导向的位点和对映选择性 C(sp3)-H 官能化是一项艰巨的挑战。我们在此报告了铱催化的伯醇衍生氨基甲酸酯的不对称 α-C(sp3)-H 硼酸化的第一个例子,通过明智地选择定向基团。获得多种手性硼酸化氨基甲酸酯,具有良好至高的对映选择性。我们还通过利用 C-B 键的高度可转化特性和氨基甲酸酯的离开能力证明了合成效用。
更新日期:2024-12-19
中文翻译:

铱催化实现掩蔽伯醇的对映选择性 α-C(sp3)–H 硼酸化
醇或掩蔽醇的官能团导向的位点和对映选择性 C(sp3)-H 官能化是一项艰巨的挑战。我们在此报告了铱催化的伯醇衍生氨基甲酸酯的不对称 α-C(sp3)-H 硼酸化的第一个例子,通过明智地选择定向基团。获得多种手性硼酸化氨基甲酸酯,具有良好至高的对映选择性。我们还通过利用 C-B 键的高度可转化特性和氨基甲酸酯的离开能力证明了合成效用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号