当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Therapeutic Efficacy of a Synthetic Brain-Targeted H2S Donor Cross-Linked Nanomicelle in Autism Spectrum Disorder Rats through Aerobic Glycolysis
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-12-18 , DOI: 10.1021/acsami.4c11663 Changmei Zhang, Lingyuan Yang, Feng Wang, Mingyuan Liu, Zehui Liu, Mingyang Zou, Lijie Wu
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-12-18 , DOI: 10.1021/acsami.4c11663 Changmei Zhang, Lingyuan Yang, Feng Wang, Mingyuan Liu, Zehui Liu, Mingyang Zou, Lijie Wu
|
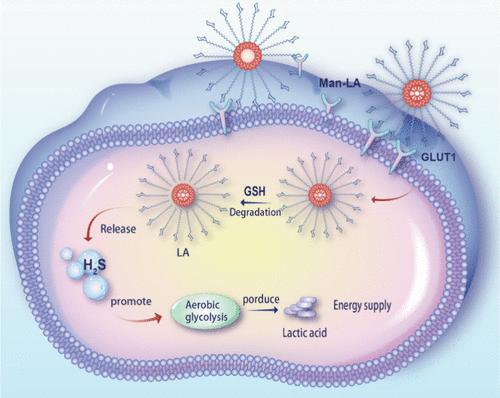
Autism spectrum disorder (ASD) is characterized by cognitive inflexibility and social deficits, with a notably limited range of brain-targeted medications, particularly in the field of nanomedicine. Herein, we introduce the brain-targeted H2S donor cross-linked nanomicelle, named mannose-PEG600-lipoic acid (Man-LA). Man-LA demonstrates enhanced stability and precise brain delivery by interacting with glucose transporter 1 (GLUT1) in astrocytes, facilitating a gradual release of H2S that is modulated by glutathione (GSH). In vivo, studies suggest that Man-LA alleviates symptoms of ASD, correlating with increased expression of aerobic glycolysis enzymes, elevated lactate production, and higher H2S levels, while preventing damage to hippocampal neurons. In vitro, Man-LA tightly binds to aldehyde dehydrogenase family 3 member B1 (Aldh3b1) in astrocytes, upregulating its expression. This interaction promotes aerobic glycolysis and enhances lactate production. These findings suggest a connection between ASD deficits and the dysregulation of astrocytic aerobic glycolysis, underscoring the role of H2S. Identifying the Aldh3b1 gene within aerobic glycolysis pathways provides a promising target for ASD treatment.
中文翻译:

合成脑靶向 H2S 供体交联纳米胶束通过有氧糖酵解对自闭症谱系障碍大鼠的治疗效果
自闭症谱系障碍 (ASD) 的特点是认知不灵活和社交缺陷,脑靶向药物的范围明显有限,尤其是在纳米医学领域。在此,我们介绍了脑靶向的 H2S 供体交联纳米胶束,命名为甘露糖-PEG600-硫辛酸 (Man-LA)。Man-LA 通过与星形胶质细胞中的葡萄糖转运蛋白 1 (GLUT1) 相互作用,促进受谷胱甘肽 (GSH) 调节的 H2S 的逐渐释放,表现出增强的稳定性和精确的脑递送。在体内,研究表明 Man-LA 可缓解 ASD 的症状,与需氧糖酵解酶表达增加、乳酸产生增加和 H2S 水平升高相关,同时防止对海马神经元的损伤。在体外,Man-LA 与星形胶质细胞中的醛脱氢酶家族 3 成员 B1 (Aldh3b1) 紧密结合,上调其表达。这种相互作用促进有氧糖酵解并增强乳酸的产生。这些发现表明 ASD 缺陷与星形胶质细胞有氧糖酵解失调之间存在联系,强调了 H2S 的作用。鉴定有氧糖酵解途径中的 Aldh3b1 基因为 ASD 治疗提供了一个有希望的靶点。
更新日期:2024-12-19
中文翻译:

合成脑靶向 H2S 供体交联纳米胶束通过有氧糖酵解对自闭症谱系障碍大鼠的治疗效果
自闭症谱系障碍 (ASD) 的特点是认知不灵活和社交缺陷,脑靶向药物的范围明显有限,尤其是在纳米医学领域。在此,我们介绍了脑靶向的 H2S 供体交联纳米胶束,命名为甘露糖-PEG600-硫辛酸 (Man-LA)。Man-LA 通过与星形胶质细胞中的葡萄糖转运蛋白 1 (GLUT1) 相互作用,促进受谷胱甘肽 (GSH) 调节的 H2S 的逐渐释放,表现出增强的稳定性和精确的脑递送。在体内,研究表明 Man-LA 可缓解 ASD 的症状,与需氧糖酵解酶表达增加、乳酸产生增加和 H2S 水平升高相关,同时防止对海马神经元的损伤。在体外,Man-LA 与星形胶质细胞中的醛脱氢酶家族 3 成员 B1 (Aldh3b1) 紧密结合,上调其表达。这种相互作用促进有氧糖酵解并增强乳酸的产生。这些发现表明 ASD 缺陷与星形胶质细胞有氧糖酵解失调之间存在联系,强调了 H2S 的作用。鉴定有氧糖酵解途径中的 Aldh3b1 基因为 ASD 治疗提供了一个有希望的靶点。






























 京公网安备 11010802027423号
京公网安备 11010802027423号