当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering a Binding Peptide for Oriented Immobilization and Efficient Bioelectrocatalytic Oxygen Reduction of Multicopper Oxidases
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-12-18 , DOI: 10.1021/acsami.4c12970 Meng Zhang, Xiufeng Wang, Weisong Liu, Xinyu Cui, Yuanming Wang, Lin Fan, Huijuan Cui, Yanbing Shen, Haiyang Cui, Lingling Zhang
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-12-18 , DOI: 10.1021/acsami.4c12970 Meng Zhang, Xiufeng Wang, Weisong Liu, Xinyu Cui, Yuanming Wang, Lin Fan, Huijuan Cui, Yanbing Shen, Haiyang Cui, Lingling Zhang
|
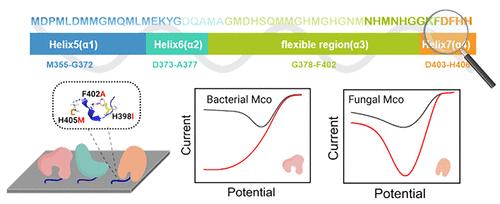
Enzymatic fuel cells (EFCs) are emerging as promising technologies in renewable energy and biomedical applications, utilizing enzyme catalysts to convert the chemical energy of renewable biomass into electrical energy, known for their high energy conversion efficiency and excellent biocompatibility. Currently, EFCs face challenges of poor stability and catalytic efficiency at the cathodes, necessitating solutions to enhance the oriented immobilization of multicopper oxidases for improved heterogeneous electron transfer efficiency. This study successfully identified a surface-binding peptide (SBP, 13 amino acids) derived from a methionine-rich fragment (MetRich, 53 amino acids) in E. coli CueO through semirational design. The first phase of engineering focused on the structural characteristics of MetRich, pinpointing fragment N394-H406 (SBP 1.0, corresponding to variant CueO-M12) as the key region dominating the binding. Subsequent site-saturation mutagenesis, combined with electrochemical screening, yielded three variants, and among them, the variant CueO-M12-1 (CueO-M12 H398I) exhibited a more uniform favorable orientation with a 1.38-fold increase in current density. Further electrocatalytic kinetics analysis revealed a significant 21.2-fold improvement in kinetics current density (Jk) compared with that of CueO-WT, leading to the development of SBP 2.0. When SBPs were fused to laccase from Bacillus pumilus (BpL) and fungal bilirubin oxidase from Myrothecium verrucaria (MvBOD), respectively, they transformed a sluggish adsorption process into a rapid and oriented one. In addition, compared with SBP 1.0, SBP 2.0 endows BpL and MvBOD with enhanced electrocatalytic capabilities for oxygen reduction and glucose/O2 EFC performance. The engineered SBPs are promising for serving as a versatile “glue” to enable the immobilization of oxidoreductases in an oriented manner, which leads to a breakthrough in bioelectrocatalysis and thereby overcoming the current bottleneck of EFCs.
中文翻译:

设计一种结合肽,用于定向固定和多铜氧化酶的高效生物电催化氧还原
酶燃料电池 (EFC) 正在成为可再生能源和生物医学应用中前景广阔的技术,它利用酶催化剂将可再生生物质的化学能转化为电能,以其高能量转换效率和出色的生物相容性而闻名。目前,EFC 面临阴极稳定性和催化效率差的挑战,需要解决方案来增强多氧化铜酶的定向固定化,以提高多相电子转移效率。本研究通过半理性设计成功鉴定了一种表面结合肽 (SBP,13 个氨基酸),来源于大肠杆菌 CueO 中富含蛋氨酸的片段 (MetRich,53 个氨基酸)。工程的第一阶段侧重于 MetRich 的结构特征,确定片段 N394-H406 (SBP 1.0,对应于变体 CueO-M12) 作为主导结合的关键区域。随后的位点饱和诱变,结合电化学筛选,产生了三种变体,其中变体 CueO-M12-1 (CueO-M12 H398I) 表现出更均匀的有利取向,电流密度增加了 1.38 倍。进一步的电催化动力学分析显示,与 CueO-WT 相比,动力学电流密度 (Jk) 显着提高了 21.2 倍,导致了 SBP 2.0 的发展。当 SBPs 分别与来自短芽孢杆菌 (BpL) 的漆酶和来自疣状芽孢杆菌的真菌胆红素氧化酶 (MvBOD) 融合时,它们将缓慢的吸附过程转变为快速和定向的过程。此外,与 SBP 1.0 相比,SBP 2.0 赋予 BpL 和 MvBOD 增强的电催化能力,用于减氧和葡萄糖/O2 EFC 性能。工程化的 SBP 有望作为一种多功能的“胶水”,能够以定向方式固定氧化还原酶,这导致了生物电催化的突破,从而克服了当前 EFC 的瓶颈。
更新日期:2024-12-18
中文翻译:

设计一种结合肽,用于定向固定和多铜氧化酶的高效生物电催化氧还原
酶燃料电池 (EFC) 正在成为可再生能源和生物医学应用中前景广阔的技术,它利用酶催化剂将可再生生物质的化学能转化为电能,以其高能量转换效率和出色的生物相容性而闻名。目前,EFC 面临阴极稳定性和催化效率差的挑战,需要解决方案来增强多氧化铜酶的定向固定化,以提高多相电子转移效率。本研究通过半理性设计成功鉴定了一种表面结合肽 (SBP,13 个氨基酸),来源于大肠杆菌 CueO 中富含蛋氨酸的片段 (MetRich,53 个氨基酸)。工程的第一阶段侧重于 MetRich 的结构特征,确定片段 N394-H406 (SBP 1.0,对应于变体 CueO-M12) 作为主导结合的关键区域。随后的位点饱和诱变,结合电化学筛选,产生了三种变体,其中变体 CueO-M12-1 (CueO-M12 H398I) 表现出更均匀的有利取向,电流密度增加了 1.38 倍。进一步的电催化动力学分析显示,与 CueO-WT 相比,动力学电流密度 (Jk) 显着提高了 21.2 倍,导致了 SBP 2.0 的发展。当 SBPs 分别与来自短芽孢杆菌 (BpL) 的漆酶和来自疣状芽孢杆菌的真菌胆红素氧化酶 (MvBOD) 融合时,它们将缓慢的吸附过程转变为快速和定向的过程。此外,与 SBP 1.0 相比,SBP 2.0 赋予 BpL 和 MvBOD 增强的电催化能力,用于减氧和葡萄糖/O2 EFC 性能。工程化的 SBP 有望作为一种多功能的“胶水”,能够以定向方式固定氧化还原酶,这导致了生物电催化的突破,从而克服了当前 EFC 的瓶颈。

































 京公网安备 11010802027423号
京公网安备 11010802027423号