当前位置:
X-MOL 学术
›
Anal. Chim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
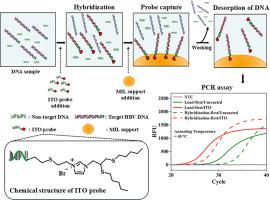
Enhancing the Interactions of Ion-Tagged Oligonucleotides and Magnetic Ionic Liquid Supports for the Sequence-Specific Extraction of Hepatitis B Virus DNA
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2024-12-18 , DOI: 10.1016/j.aca.2024.343563 Seong-Soo Lee, Derek R. Eitzmann, Jared L. Anderson
更新日期:2024-12-19
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2024-12-18 , DOI: 10.1016/j.aca.2024.343563 Seong-Soo Lee, Derek R. Eitzmann, Jared L. Anderson
|






























 京公网安备 11010802027423号
京公网安备 11010802027423号