当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface reconstruction of copper(Ⅰ) sulfide electrode regulated electroreduction CO2 selectivity
Applied Surface Science ( IF 6.3 ) Pub Date : 2024-12-18 , DOI: 10.1016/j.apsusc.2024.162139 Qin Ding, Jianhua Lin, Xiaoyu Li, Yu Dong, Yongchun Bao, Huazhong Liang, Quan Zhuang, Jinghai Liu, Yin Wang
Applied Surface Science ( IF 6.3 ) Pub Date : 2024-12-18 , DOI: 10.1016/j.apsusc.2024.162139 Qin Ding, Jianhua Lin, Xiaoyu Li, Yu Dong, Yongchun Bao, Huazhong Liang, Quan Zhuang, Jinghai Liu, Yin Wang
|
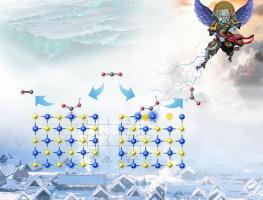
Carbon dioxide (CO2) electroreduction to valuable chemicals is a promising approach for efficient carbon utilization. Herein, we propose an in situ electrochemical reconstruction method to regulate the selectivity of electroreduction of CO2 into CO or HCOOH using a copper sulfide-loaded copper foam (Cu2S/CF) self-supporting electrode. Cu2S/CF under the applied potential of −0.2 V versus reversible hydrogen electrode (vs. RHE) maintain as the intial one, and Cu+ are partially reduced to Cu0 under the applied potential of −0.4 V vs. RHE forming vacancy S defects (VS) in Cu2S. Notably, the Cu2S/CF achieve a potential regulable electroreduction CO2 into CO with faradaic efficiency (FE) of 84.6 % at the applied potential of −0.2 V vs. reversible hydrogen electrode (RHE) and HCOOH with FE of 81.6 % at −0.4 V vs. RHE. Density functional theory (DFT) calculations reveal the different CO2 adsorption configurations of catalytic centers resulting in the difference on the reduction products. Saturation Cu site on Cu2S surface favours to adsorb CO2 with end-on configuration of *COOH for CO production, and unsaturation Cu site on Cu2S-VS surface prefers to the bridge configuration of *O*COH for HCOOH synthesis.
中文翻译:

硫化铜(I.)电极表面重构调节电还原CO2选择性
二氧化碳 (CO 2 ) 电还原成有价值的化学品是一种很有前途的碳有效利用方法。在此,我们提出了一种原位电化学重建方法,以使用负载硫化铜的泡沫铜 (Cu 2 S/CF) 自支撑电极调节 CO 电还原 2 为 CO 或 HCOOH 的选择性。与 RHE 相比,在 -0.2 V 的施加电位下,Cu 2 S/CF 保持为初始电位,在 -0.4 V 与 RHE 的施加电位 0 下,Cu + 部分还原为 Cu 2 S 中形成空位 S 缺陷 (V S )。 2 2 与可逆氢电极 (RHE) 相比,在 -0.2 V 的施加电位下,法拉第效率 (FE) 为 84.6%,HCOOH 在 -0.4 V 与 RHE 的 FE 为 81.6%。密度泛函理论 (DFT) 计算揭示了催化中心的不同 CO 2 吸附构型,从而导致还原产物的差异。Cu 2 S 表面的饱和 Cu 位点有利于以 *COOH 的端接构型吸附 CO 2 以产生 CO,而 Cu 2 S-V S 表面上的不饱和 Cu 位点优于 *O*COH 的桥构型用于 HCOOH 合成。
更新日期:2024-12-19
中文翻译:

硫化铜(I.)电极表面重构调节电还原CO2选择性
二氧化碳 (CO 2 ) 电还原成有价值的化学品是一种很有前途的碳有效利用方法。在此,我们提出了一种原位电化学重建方法,以使用负载硫化铜的泡沫铜 (Cu 2 S/CF) 自支撑电极调节 CO 电还原 2 为 CO 或 HCOOH 的选择性。与 RHE 相比,在 -0.2 V 的施加电位下,Cu 2 S/CF 保持为初始电位,在 -0.4 V 与 RHE 的施加电位 0 下,Cu + 部分还原为 Cu 2 S 中形成空位 S 缺陷 (V S )。 2 2 与可逆氢电极 (RHE) 相比,在 -0.2 V 的施加电位下,法拉第效率 (FE) 为 84.6%,HCOOH 在 -0.4 V 与 RHE 的 FE 为 81.6%。密度泛函理论 (DFT) 计算揭示了催化中心的不同 CO 2 吸附构型,从而导致还原产物的差异。Cu 2 S 表面的饱和 Cu 位点有利于以 *COOH 的端接构型吸附 CO 2 以产生 CO,而 Cu 2 S-V S 表面上的不饱和 Cu 位点优于 *O*COH 的桥构型用于 HCOOH 合成。






























 京公网安备 11010802027423号
京公网安备 11010802027423号