当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Overcoming radiation-induced PD-L1 upregulation by novel gadolinium-palladium nanoplatforms for enhanced tumor radio-immunotherapy
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-12-18 , DOI: 10.1016/j.cej.2024.158754
Kai Guo, Hui Liu, Mengmeng Zhang, Nengyi Ni, Mengyao Mu, Ke Ren, Jiahui Chen, Qing Fan, Xueli Xu, Xiao Sun, Ximing Wang
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-12-18 , DOI: 10.1016/j.cej.2024.158754
Kai Guo, Hui Liu, Mengmeng Zhang, Nengyi Ni, Mengyao Mu, Ke Ren, Jiahui Chen, Qing Fan, Xueli Xu, Xiao Sun, Ximing Wang
|
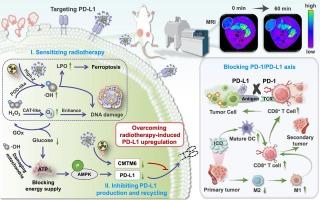
Low-dose radiotherapy can lead to upregulation of PD-L1 expression in tumor cells, limiting the effectiveness of radio-immunotherapy. Currently, anti-PD-L1 drugs only disrupt the interaction of the PD-1/PD-L1 axis on the cell surface, without considering the immune regulatory ability of intracellular PD-L1. Additionally, antibody-bound PD-L1 can lead to internalization and recycling, allowing tumor cells to regain immune suppression. In this study, a novel PD-L1-affibody (ZPD-L1 )-grafted gadolinium-palladium nanoplatform incorporated with glucose oxidase (GOx) (GPGP) was fabricated to improve tumor-targeted magnetic resonance imaging (MRI) and synergistic radio-immunotherapy. GPGP with catalase- and peroxidase-like activity could catalyze the generation of oxygen to relieve tumor hypoxia and the generation of •OH to boost ferroptosis, respectively. GOx could enhance the nanoplatform’s dual nanozyme activity in a cascade, further sensitizing radiotherapy and intensifying subsequent immunogenic cell death. Crucially, GPGP induced AMPK phosphorylation and downregulated CMTM6, inhibiting intracellular PD-L1 production and blocking its transport to the cell membrane, thereby overcoming low-dose radiotherapy-induced PD-L1 upregulation. Systemic delivery of GPGP also demonstrated notable MRI contrast enhancement, providing precise imaging guidance for determining the optimal timing for radiotherapy. Therefore, GPGP has shown great potential in achieving effective MRI and collaborative tumor radio-immunotherapy.
中文翻译:

通过新型钆-钯纳米平台克服辐射诱导的 PD-L1 上调,以增强肿瘤放射免疫治疗
低剂量放疗可导致肿瘤细胞中 PD-L1 表达上调,从而限制放疗免疫治疗的有效性。目前,抗 PD-L1 药物仅破坏细胞表面 PD-1/PD-L1 轴的相互作用,而没有考虑细胞内 PD-L1 的免疫调节能力。此外,抗体结合的 PD-L1 可导致内化和再循环,使肿瘤细胞重新获得免疫抑制。在这项研究中,制造了一种新型 PD-L1-affibody (ZPD-L1) 接枝钆-钯纳米平台,掺入葡萄糖氧化酶 (GOx) (GPGP),以改善肿瘤靶向磁共振成像 (MRI) 和协同放射免疫治疗。具有过氧化氢酶和过氧化物酶样活性的 GPGP 可以分别催化氧气的产生以缓解肿瘤缺氧和 •OH 的产生以促进铁死亡。GOx 可以在级联反应中增强纳米平台的双纳米酶活性,进一步敏化放疗并加剧随后的免疫原性细胞死亡。至关重要的是,GPGP 诱导 AMPK 磷酸化并下调 CMTM6,抑制细胞内 PD-L1 的产生并阻断其向细胞膜的转运,从而克服低剂量放疗诱导的 PD-L1 上调。GPGP 的全身递送还显示出显着的 MRI 对比增强,为确定放疗的最佳时机提供了精确的成像指导。因此,GPGP 在实现有效的 MRI 和协作肿瘤放射免疫治疗方面显示出巨大潜力。
更新日期:2024-12-18
中文翻译:

通过新型钆-钯纳米平台克服辐射诱导的 PD-L1 上调,以增强肿瘤放射免疫治疗
低剂量放疗可导致肿瘤细胞中 PD-L1 表达上调,从而限制放疗免疫治疗的有效性。目前,抗 PD-L1 药物仅破坏细胞表面 PD-1/PD-L1 轴的相互作用,而没有考虑细胞内 PD-L1 的免疫调节能力。此外,抗体结合的 PD-L1 可导致内化和再循环,使肿瘤细胞重新获得免疫抑制。在这项研究中,制造了一种新型 PD-L1-affibody (ZPD-L1) 接枝钆-钯纳米平台,掺入葡萄糖氧化酶 (GOx) (GPGP),以改善肿瘤靶向磁共振成像 (MRI) 和协同放射免疫治疗。具有过氧化氢酶和过氧化物酶样活性的 GPGP 可以分别催化氧气的产生以缓解肿瘤缺氧和 •OH 的产生以促进铁死亡。GOx 可以在级联反应中增强纳米平台的双纳米酶活性,进一步敏化放疗并加剧随后的免疫原性细胞死亡。至关重要的是,GPGP 诱导 AMPK 磷酸化并下调 CMTM6,抑制细胞内 PD-L1 的产生并阻断其向细胞膜的转运,从而克服低剂量放疗诱导的 PD-L1 上调。GPGP 的全身递送还显示出显着的 MRI 对比增强,为确定放疗的最佳时机提供了精确的成像指导。因此,GPGP 在实现有效的 MRI 和协作肿瘤放射免疫治疗方面显示出巨大潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号