当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing Chordoma Radiotherapy: Ta@PVP Nanoparticles as Potent Radiosensitizers
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-12-18 , DOI: 10.1021/acsami.4c19601 Wancheng Li, Shuheng Zhang, Linhong Liu, Mingxuan Li, Jinfeng He, Qingguo Meng, Jiali Kang, Dabiao Zhou, Liang Gao, Jiwei Bai, Zhanjun Gu, Fuping Gao
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-12-18 , DOI: 10.1021/acsami.4c19601 Wancheng Li, Shuheng Zhang, Linhong Liu, Mingxuan Li, Jinfeng He, Qingguo Meng, Jiali Kang, Dabiao Zhou, Liang Gao, Jiwei Bai, Zhanjun Gu, Fuping Gao
|
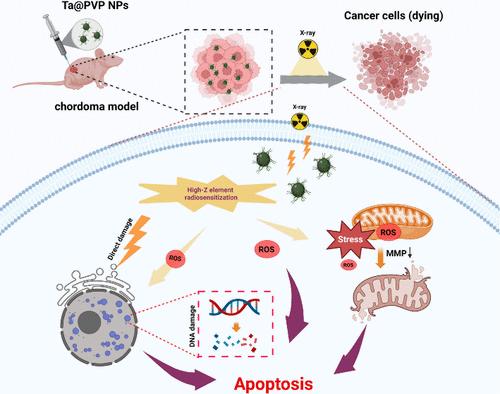
Surgical resection and high-dose radiotherapy constitute the standard therapeutic approaches for chordoma. However, the efficacy of radiotherapy is often compromised by the tumor microenvironment’s hypoxic conditions, which confer radiation resistance, and by the potential damage to adjacent spinal cord and neural structures from elevated radiation doses. To address these challenges, we employed high biocompatible poly(vinylpyrrolidone)-modified tantalum nanoparticles (Ta@PVP NPs) as a potent radiosensitizer to augment the radiotherapy sensitivity of chordoma. Upon exposure to X-ray irradiation, Ta@PVP NPs demonstrated the capability to efficiently deposit X-ray radiation energy within the tumor microenvironment, subsequently generating reactive oxygen species (ROS) that induce oxidative stress in the tumor. Both in vitro and in vivo experiments revealed that Ta@PVP NPs significantly enhanced the cytotoxic effects of X-ray, thereby markedly inhibiting the proliferation of chordoma cells and impeding tumor growth. This study explored the radiosensitization potential of Ta@PVP NPs in the context of chordoma, highlighting the application of radiosensitizers as a promising strategy to augment the efficacy of chordoma radiotherapy.
中文翻译:

增强脊索瘤放射治疗:Ta@PVP纳米颗粒作为强效放射增敏剂
手术切除和大剂量放疗是脊索瘤的标准治疗方法。然而,放疗的疗效通常会受到肿瘤微环境的缺氧条件的影响,这些条件赋予了辐射抗性,并且由于辐射剂量增加对邻近脊髓和神经结构的潜在损害。为了应对这些挑战,我们采用了高生物相容性聚(乙烯基吡咯烷酮)改性钽纳米颗粒 (Ta@PVP NPs) 作为有效的放射增敏剂,以增强脊索瘤的放射治疗敏感性。在暴露于 X 射线照射下,Ta@PVP NPs 表现出在肿瘤微环境中有效沉积 X 射线辐射能量的能力,随后产生活性氧 (ROS),在肿瘤中诱导氧化应激。体外和体内实验均表明,Ta@PVP NPs 显着增强了 X 射线的细胞毒作用,从而显着抑制脊索瘤细胞的增殖并阻碍肿瘤生长。本研究探讨了 Ta@PVP NPs 在脊索瘤背景下的放射增敏潜力,强调了放射增敏剂作为增强脊索瘤放疗疗效的一种有前途的策略。
更新日期:2024-12-19
中文翻译:

增强脊索瘤放射治疗:Ta@PVP纳米颗粒作为强效放射增敏剂
手术切除和大剂量放疗是脊索瘤的标准治疗方法。然而,放疗的疗效通常会受到肿瘤微环境的缺氧条件的影响,这些条件赋予了辐射抗性,并且由于辐射剂量增加对邻近脊髓和神经结构的潜在损害。为了应对这些挑战,我们采用了高生物相容性聚(乙烯基吡咯烷酮)改性钽纳米颗粒 (Ta@PVP NPs) 作为有效的放射增敏剂,以增强脊索瘤的放射治疗敏感性。在暴露于 X 射线照射下,Ta@PVP NPs 表现出在肿瘤微环境中有效沉积 X 射线辐射能量的能力,随后产生活性氧 (ROS),在肿瘤中诱导氧化应激。体外和体内实验均表明,Ta@PVP NPs 显着增强了 X 射线的细胞毒作用,从而显着抑制脊索瘤细胞的增殖并阻碍肿瘤生长。本研究探讨了 Ta@PVP NPs 在脊索瘤背景下的放射增敏潜力,强调了放射增敏剂作为增强脊索瘤放疗疗效的一种有前途的策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号