当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 2-Phosphonylated C3 Spirocyclic Indolines via a Dearomatization–Spirocyclization–Nucleophilic Addition Tandem Approach of Indolyl-ynones with Phosphine Oxides
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-12-18 , DOI: 10.1021/acs.joc.4c02364 Jiongjiong Duan, Yi Cao, Huanping Xie, Yongqi Yu, Heyun Sheng, Weiguang Kong, Ting Li
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-12-18 , DOI: 10.1021/acs.joc.4c02364 Jiongjiong Duan, Yi Cao, Huanping Xie, Yongqi Yu, Heyun Sheng, Weiguang Kong, Ting Li
|
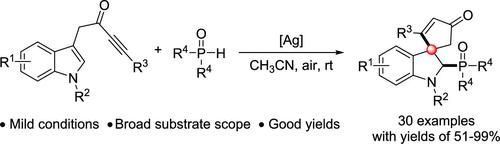
Due to its unique three-dimensional (3D) configuration and great application potential in medicinal chemistry and synthetic community, chemists have been pursuing concise and efficient methods to synthesize C3 spirocyclic indoline derivatives. In this work, a dearomatization–spirocyclization–nucleophilic addition tandem approach was developed to realize the synthesis of 2-phosphonylated C3 spirocyclic indolines with indolyl-ynones and phosphine oxides as reactants; mild conditions, broad substrate scope, and good yields are characteristics of this transformation.
中文翻译:

通过吲哚基-炙酮与磷化氢的脱芳烃化-螺环化-亲核加成串联方法合成 2-膦酰化 C3 螺环吲哚啉
由于其独特的三维 (3D) 构型以及在药物化学和合成界的巨大应用潜力,化学家们一直在寻求简洁有效的方法来合成 C3 螺环吲哚啉衍生物。在这项工作中,开发了一种脱芳烃化-螺环化-亲核加成串联方法,以实现以吲哚基-伊酮和磷化氢氧化物为反应物的 2-膦酰化 C3 螺环吲哚啉的合成;温和的条件、广泛的底物范围和良好的产量是这种转变的特点。
更新日期:2024-12-18
中文翻译:

通过吲哚基-炙酮与磷化氢的脱芳烃化-螺环化-亲核加成串联方法合成 2-膦酰化 C3 螺环吲哚啉
由于其独特的三维 (3D) 构型以及在药物化学和合成界的巨大应用潜力,化学家们一直在寻求简洁有效的方法来合成 C3 螺环吲哚啉衍生物。在这项工作中,开发了一种脱芳烃化-螺环化-亲核加成串联方法,以实现以吲哚基-伊酮和磷化氢氧化物为反应物的 2-膦酰化 C3 螺环吲哚啉的合成;温和的条件、广泛的底物范围和良好的产量是这种转变的特点。






























 京公网安备 11010802027423号
京公网安备 11010802027423号