当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expanding the Repertoire of Large Scaffolds with Syn and Anti Macrocyclic Metacyclophanes
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-12-17 , DOI: 10.1021/acs.joc.4c02295 Liang-Yu Chen, Udayan Chaudhury, Shengkai Wei, Junqi Li
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-12-17 , DOI: 10.1021/acs.joc.4c02295 Liang-Yu Chen, Udayan Chaudhury, Shengkai Wei, Junqi Li
|
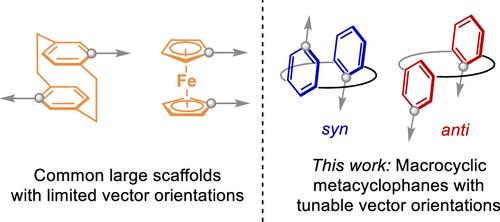
Understanding how changes in structure translate to changes in molecular shape is key to catalyst optimization and molecular design in medicinal chemistry and materials. One key contributor to the molecular shape is the relative orientation of substituents on a scaffold. Macrocyclic metacyclophanes display their two arenes in a parallel or antiparallel fashion, resulting in anti or syn conformations that lead to disparate relative orientations of the aryl substituents. This work reports the synthesis of new 14- and 16-membered metacyclophanes and the elucidation of their anti/syn preferences by 1H NMR and computational conformational analysis. Most metacyclophanes studied herein display a strong anti or syn preference and, thus, have well-defined substituent orientations. We propose that anti/syn conformational preferences arise from the minimization of torsional strain along the backbone of the macrocycle, which leads to the prediction that metacyclophanes with remote aryl substituents will adopt the same conformation as their unsubstituted counterparts. Exit vector analysis also reveals that anti-metacyclophanes project their substituents into regions in three-dimensional space that are not accessed by other common large scaffolds, e.g., [2.2]paracyclophanes and ferrocenes. This work also demonstrates how ring size and functional groups, two parameters commonly optimized in macrocycle design, can be used to tune molecular shape.
中文翻译:

使用 Syn 和抗大环 Metacyclophanes 扩展大型支架的库
了解结构变化如何转化为分子形状的变化是药物化学和材料中催化剂优化和分子设计的关键。分子形状的一个关键因素是支架上取代基的相对取向。大环间环烷以平行或反平行的方式显示它们的两个芳烃,导致反或同构象,导致芳基取代基的不同相对取向。这项工作报道了新的 14 元和 16 元元环烷的合成,以及通过 1H NMR 和计算构象分析阐明它们的抗/syn 偏好。本文研究的大多数 metacyclophanes 显示出强烈的抗或 syn 偏好性,因此具有明确的取代基取向。我们提出 anti/syn 构象偏好源于沿大环主干的扭转应变最小化,这导致预测具有远程芳基取代基的 metacyclophanes 将采用与其未取代的对应物相同的构象。出口向量分析还显示,抗偏环烷将其取代基投射到三维空间中其他常见的大型支架(例如 [2.2]对环烷和二茂铁)无法进入的区域。这项工作还展示了如何使用环大小和官能团(大环设计中通常优化的两个参数)来调整分子形状。
更新日期:2024-12-18
中文翻译:

使用 Syn 和抗大环 Metacyclophanes 扩展大型支架的库
了解结构变化如何转化为分子形状的变化是药物化学和材料中催化剂优化和分子设计的关键。分子形状的一个关键因素是支架上取代基的相对取向。大环间环烷以平行或反平行的方式显示它们的两个芳烃,导致反或同构象,导致芳基取代基的不同相对取向。这项工作报道了新的 14 元和 16 元元环烷的合成,以及通过 1H NMR 和计算构象分析阐明它们的抗/syn 偏好。本文研究的大多数 metacyclophanes 显示出强烈的抗或 syn 偏好性,因此具有明确的取代基取向。我们提出 anti/syn 构象偏好源于沿大环主干的扭转应变最小化,这导致预测具有远程芳基取代基的 metacyclophanes 将采用与其未取代的对应物相同的构象。出口向量分析还显示,抗偏环烷将其取代基投射到三维空间中其他常见的大型支架(例如 [2.2]对环烷和二茂铁)无法进入的区域。这项工作还展示了如何使用环大小和官能团(大环设计中通常优化的两个参数)来调整分子形状。






























 京公网安备 11010802027423号
京公网安备 11010802027423号