当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron-Catalyzed Miyaura Borylation of Aryl Chlorides and Triflates
Organic Letters ( IF 4.9 ) Pub Date : 2024-12-17 , DOI: 10.1021/acs.orglett.4c04171 Patrick Daley-Dee, James Clarke, Sebastien Monfette, Robin B. Bedford
Organic Letters ( IF 4.9 ) Pub Date : 2024-12-17 , DOI: 10.1021/acs.orglett.4c04171 Patrick Daley-Dee, James Clarke, Sebastien Monfette, Robin B. Bedford
|
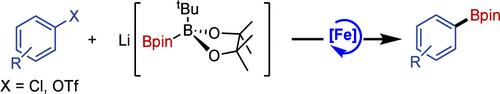
Simple aryl chlorides represent challenging substrates in iron-catalyzed borylation. A combination of Li[B(tBu)pin-Bpin] as the borylating reagent and a catalyst formed in situ from iron(II) triflate and the commercially available N-heterocyclic carbene ligand, IMes, gives significantly improved activity and a much broader scope than previously reported iron-based catalysts. Iron triflate is also a good precatalyst for the borylation of aryl triflates─a previously unreported transformation─and in these cases the IMes ligand is not required.
中文翻译:

铁催化芳基氯化物和三氟甲磺酸酯的 Miyaura 硼化反应
简单的芳基氯化物是铁催化硼酸化反应中具有挑战性的底物。作为硼化试剂的 Li[B(tBu)pin-Bpin] 与由三氟甲磺酸铁原位形成的催化剂和市售的 N-杂环卡宾配体 IMes 的组合,与以前报道的铁基催化剂相比,活性显著提高,范围更广。三氟甲磺酸铁也是芳基三氟甲磺酸铁硼化的良好预催化剂(一种以前未报道的转化),在这些情况下不需要 IMes 配体。
更新日期:2024-12-18
中文翻译:

铁催化芳基氯化物和三氟甲磺酸酯的 Miyaura 硼化反应
简单的芳基氯化物是铁催化硼酸化反应中具有挑战性的底物。作为硼化试剂的 Li[B(tBu)pin-Bpin] 与由三氟甲磺酸铁原位形成的催化剂和市售的 N-杂环卡宾配体 IMes 的组合,与以前报道的铁基催化剂相比,活性显著提高,范围更广。三氟甲磺酸铁也是芳基三氟甲磺酸铁硼化的良好预催化剂(一种以前未报道的转化),在这些情况下不需要 IMes 配体。






























 京公网安备 11010802027423号
京公网安备 11010802027423号