Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Localized Microrobotic Delivery of Enzyme‐Responsive Hydrogel‐Immobilized Therapeutics to Suppress Triple‐Negative Breast Cancer
Small ( IF 13.0 ) Pub Date : 2024-12-18 , DOI: 10.1002/smll.202408813 Mingzhen Tian, Meysam Keshavarz, Ali Anil Demircali, Bing Han, Guang‐Zhong Yang
Small ( IF 13.0 ) Pub Date : 2024-12-18 , DOI: 10.1002/smll.202408813 Mingzhen Tian, Meysam Keshavarz, Ali Anil Demircali, Bing Han, Guang‐Zhong Yang
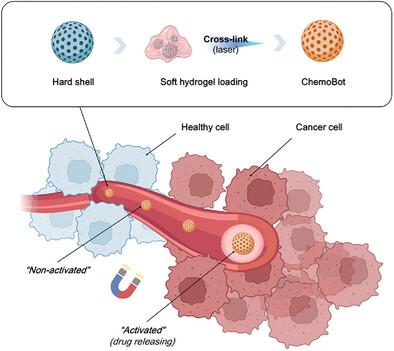
|
Triple‐negative breast cancer (TNBC), characterized by its aggressive metastatic propensity and lack of effective targeted therapeutic options, poses a major challenge in oncological management. A proof‐of‐concept neoadjuvant strategy aimed at inhibiting TNBC tumor growth and mitigating metastasis through a localized delivery of chemotherapeutics is reported in this paper. This approach addresses the limitations in payload capacity and stimuli responsiveness commonly associated with microrobotics in oncology. A hydrogel‐based system is developed for the immobilization of chemotherapeutic agents, subsequently encapsulated within magnetically responsive microrobots. This design leverages external magnetic fields to facilitate the precise navigation and localization of the therapeutic agents directly to the tumor site. The efficacy of this approach is demonstrated in an animal model, in which a significant 14‐fold reduction in tumor size and suppression of metastasis to critical organs such as the liver and lungs are observed. Crucially, the drug release mechanism is engineered to be responsive to the tumor microenvironment and is regulated by the overexpression of the enzymatic activity of matrix metalloproteinases (MMP2 and MMP9) in TNBC tumors, triggering the degradation of the hydrogel matrix, leading to controlled release of the immobilized therapeutic drug. This ensures that the therapeutic action is localized, reducing systemic toxicity and enhancing treatment efficacy. These findings suggest that this neoadjuvant approach holds promise for broader applications in other cancer types.
中文翻译:

酶反应性水凝胶固定化疗法的局部微机器人递送以抑制三阴性乳腺癌
三阴性乳腺癌 (TNBC) 的特点是其侵袭性转移倾向和缺乏有效的靶向治疗选择,对肿瘤学管理构成重大挑战。本文报道了一种概念验证新辅助策略,旨在通过化疗药物的局部递送来抑制 TNBC 肿瘤生长和减轻转移。这种方法解决了肿瘤学中通常与微型机器人相关的有效载荷能力和刺激响应性的局限性。开发了一种基于水凝胶的系统,用于固定化疗药物,随后封装在磁响应微型机器人中。这种设计利用外部磁场来促进治疗剂直接精确导航和定位到肿瘤部位。这种方法的有效性在动物模型中得到了证明,其中观察到肿瘤大小显着减小了 14 倍,并抑制了向肝脏和肺等关键器官的转移。至关重要的是,药物释放机制被设计为对肿瘤微环境做出反应,并受 TNBC 肿瘤中基质金属蛋白酶 (MMP2 和 MMP9) 酶活性的过表达调节,触发水凝胶基质的降解,导致固定治疗药物的受控释放。这确保了治疗作用是局部的,减少了全身毒性并增强了治疗效果。这些发现表明,这种新辅助方法有望在其他癌症类型中得到更广泛的应用。
更新日期:2024-12-18
中文翻译:

酶反应性水凝胶固定化疗法的局部微机器人递送以抑制三阴性乳腺癌
三阴性乳腺癌 (TNBC) 的特点是其侵袭性转移倾向和缺乏有效的靶向治疗选择,对肿瘤学管理构成重大挑战。本文报道了一种概念验证新辅助策略,旨在通过化疗药物的局部递送来抑制 TNBC 肿瘤生长和减轻转移。这种方法解决了肿瘤学中通常与微型机器人相关的有效载荷能力和刺激响应性的局限性。开发了一种基于水凝胶的系统,用于固定化疗药物,随后封装在磁响应微型机器人中。这种设计利用外部磁场来促进治疗剂直接精确导航和定位到肿瘤部位。这种方法的有效性在动物模型中得到了证明,其中观察到肿瘤大小显着减小了 14 倍,并抑制了向肝脏和肺等关键器官的转移。至关重要的是,药物释放机制被设计为对肿瘤微环境做出反应,并受 TNBC 肿瘤中基质金属蛋白酶 (MMP2 和 MMP9) 酶活性的过表达调节,触发水凝胶基质的降解,导致固定治疗药物的受控释放。这确保了治疗作用是局部的,减少了全身毒性并增强了治疗效果。这些发现表明,这种新辅助方法有望在其他癌症类型中得到更广泛的应用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号