当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into Sustainable Nitrogen Fixation by Gas-phase Spectroscopic Measurements and Global Modeling of Reaction Intermediates in Humid Nitrogen Plasma
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-12-18 , DOI: 10.1021/acssuschemeng.4c05771 Ananthanarasimhan Jayanarasimhan, Robert Pierrard, Sean M. Peyres, Shurik Yatom, Davide Curreli, R. Mohan Sankaran
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-12-18 , DOI: 10.1021/acssuschemeng.4c05771 Ananthanarasimhan Jayanarasimhan, Robert Pierrard, Sean M. Peyres, Shurik Yatom, Davide Curreli, R. Mohan Sankaran
|
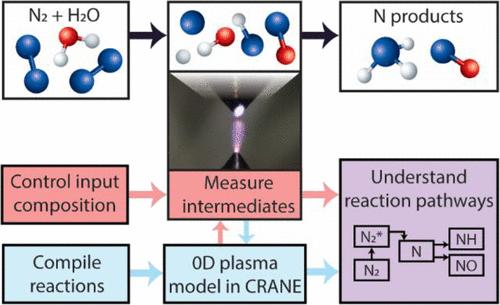
There is growing interest in reacting molecular nitrogen and water to sustainably synthesize fixed forms of nitrogen such as ammonia. In particular, low-temperature plasmas can activate these relatively inert feedstocks at or near room temperature without a catalyst. However, because of the enhanced reactivity and nonequilibrium chemistry, a diverse range of products is formed, and the underlying reaction mechanisms are exceedingly complex. In this work, we studied a simplified reactor consisting of a gaseous plasma containing controlled mixtures of nitrogen gas and water vapor. Densities of key chemical species such as N, H, OH, NH, and NO were measured by emission and laser-based spectroscopy as a function of the relative humidity. A global model was constructed and the reaction network was validated by comparing calculated species densities with experiments. We discover that N, a key initial intermediate for ammonia, strongly decreases in the presence of water vapor, and as a result, ammonia formation becomes limited at high relative humidity. This decrease is surprisingly not because N itself reacts, but because one of its main sources, an excited molecular nitrogen state, is reacted away. In addition, oxidation pathways for nitrogen, which lead to NO and related products, are found to be favored over reduction pathways because the corresponding reverse reactions are less significant. Together, this understanding helps explain previously reported observations of selectivity toward nitrogen oxides over ammonia, particularly at higher relative humidities.
中文翻译:

通过气相光谱测量和湿氮等离子体中反应中间体的全局建模,深入了解可持续固氮
人们对分子氮和水反应以可持续合成固定形式的氮(如氨)的兴趣日益浓厚。特别是,低温等离子体可以在室温或接近室温的情况下激活这些相对惰性的原料,而无需催化剂。然而,由于反应性和非平衡化学的增强,形成了多种产物,并且潜在的反应机制极其复杂。在这项工作中,我们研究了一种简化的反应器,该反应器由含有氮气和水蒸气受控混合物的气态等离子体组成。通过发射和基于激光的光谱法测量关键化学物质(如 N、H、OH、NH 和 NO)的密度,作为相对湿度的函数。构建了一个全局模型,并通过将计算的物质密度与实验进行比较来验证反应网络。我们发现,N 是氨的关键初始中间体,在水蒸气存在下会强烈减少,因此,在高相对湿度下,氨的形成变得有限。令人惊讶的是,这种减少不是因为 N 本身发生了反应,而是因为它的主要来源之一,即激发的分子氮态,被反应掉了。此外,由于相应的逆反应不太重要,因此发现导致 NO 和相关产物的氮的氧化途径优于还原途径。总之,这种理解有助于解释以前报道的对氮氧化物优于氨的选择性观察结果,尤其是在较高的相对湿度下。
更新日期:2024-12-18
中文翻译:

通过气相光谱测量和湿氮等离子体中反应中间体的全局建模,深入了解可持续固氮
人们对分子氮和水反应以可持续合成固定形式的氮(如氨)的兴趣日益浓厚。特别是,低温等离子体可以在室温或接近室温的情况下激活这些相对惰性的原料,而无需催化剂。然而,由于反应性和非平衡化学的增强,形成了多种产物,并且潜在的反应机制极其复杂。在这项工作中,我们研究了一种简化的反应器,该反应器由含有氮气和水蒸气受控混合物的气态等离子体组成。通过发射和基于激光的光谱法测量关键化学物质(如 N、H、OH、NH 和 NO)的密度,作为相对湿度的函数。构建了一个全局模型,并通过将计算的物质密度与实验进行比较来验证反应网络。我们发现,N 是氨的关键初始中间体,在水蒸气存在下会强烈减少,因此,在高相对湿度下,氨的形成变得有限。令人惊讶的是,这种减少不是因为 N 本身发生了反应,而是因为它的主要来源之一,即激发的分子氮态,被反应掉了。此外,由于相应的逆反应不太重要,因此发现导致 NO 和相关产物的氮的氧化途径优于还原途径。总之,这种理解有助于解释以前报道的对氮氧化物优于氨的选择性观察结果,尤其是在较高的相对湿度下。































 京公网安备 11010802027423号
京公网安备 11010802027423号