Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvent-Driven Na Storage in SnS2 Anodes: Atomistic Simulation-Guided Strategies for Reversible Reactions, Solid Electrolyte Interphase, and Morphological Transformation
ACS Nano ( IF 15.8 ) Pub Date : 2024-12-18 , DOI: 10.1021/acsnano.4c13669 Young-Hoon Kim, Joo-Yeon Moon, Yeong-In Yoon, Jae-Chul Lee, Yong-Seok Choi
ACS Nano ( IF 15.8 ) Pub Date : 2024-12-18 , DOI: 10.1021/acsnano.4c13669 Young-Hoon Kim, Joo-Yeon Moon, Yeong-In Yoon, Jae-Chul Lee, Yong-Seok Choi
|
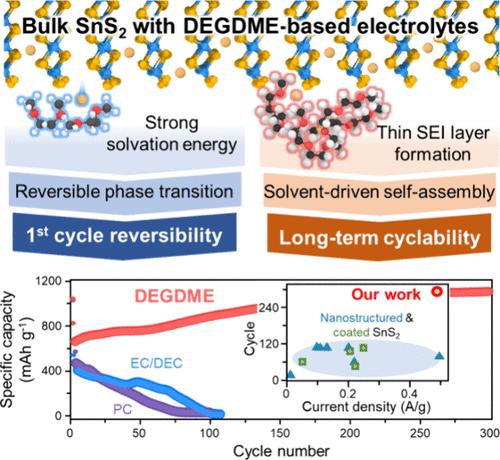
Crystalline SnS2 accommodates Na ions through intercalation–conversion–alloying (ICA) reactions, exhibiting a natural potential for high energy storage, while its layered structure facilitates rapid charging. However, these intrinsic advantages are not fully realized in practical battery applications. Herein, utilizing an innovative integration of machine-learning-based thermodynamics, artificial-neural-network-assisted molecular dynamics, and density functional theory, specific solvents are demonstrated to effectively tailor the reaction pathways. This strategy not only steers phase transition pathways but also significantly reduces the formation of the solid electrolyte interphase (SEI), which is a common issue in recent battery research. These characteristics of solvents enable reversible ICA reactions and also aid the transformation of microsized SnS2 particles into 3D porous nanostructures with minimal SEI formation. The performance of our Na–SnS2 half-cells achieve 1100 mAh g–1 (97% of the theoretical capacity) at 0.5 C, placing them among the top performers for Na storage. By moving beyond the traditional view of electrolyte solvents as a simple medium for ion transport, this work highlights the critical impact of solvent selection on enabling reversible reactions and morphological transformation of SnS2 anodes with minimal SEI formation and setting benchmarks for anode performance in energy storage systems based on ICA reactions.
中文翻译:

SnS2 负极中的溶剂驱动 Na 储存:可逆反应、固体电解质界面和形态转变的原子模拟指导策略
结晶 SnS2 通过插层-转化-合金化 (ICA) 反应容纳钠离子,表现出高能量存储的天然潜力,而其层状结构有助于快速充电。然而,这些内在优势在实际电池应用中并未完全实现。本文利用基于机器学习的热力学、人工神经网络辅助分子动力学和密度泛函理论的创新集成,证明了特定溶剂可以有效地定制反应途径。这种策略不仅控制了相变途径,还显著减少了固体电解质界面 (SEI) 的形成,这是最近电池研究中的一个常见问题。溶剂的这些特性使可逆的 ICA 反应成为可能,还有助于将微尺寸 SnS2 颗粒转化为 3D 多孔纳米结构,同时形成最少的 SEI。我们的 Na–SnS2 半电池在 0.5 C 下的性能达到 1100 mAh g–1(理论容量的 97%),使其成为 Na 存储性能最佳的产品之一。通过超越将电解质溶剂视为离子传输简单介质的传统观点,这项工作强调了溶剂选择对实现 SnS2 阳极的可逆反应和形态转变的关键影响,同时形成最小的 SEI,并为基于 ICA 反应的储能系统中的阳极性能设定了基准。
更新日期:2024-12-18
中文翻译:

SnS2 负极中的溶剂驱动 Na 储存:可逆反应、固体电解质界面和形态转变的原子模拟指导策略
结晶 SnS2 通过插层-转化-合金化 (ICA) 反应容纳钠离子,表现出高能量存储的天然潜力,而其层状结构有助于快速充电。然而,这些内在优势在实际电池应用中并未完全实现。本文利用基于机器学习的热力学、人工神经网络辅助分子动力学和密度泛函理论的创新集成,证明了特定溶剂可以有效地定制反应途径。这种策略不仅控制了相变途径,还显著减少了固体电解质界面 (SEI) 的形成,这是最近电池研究中的一个常见问题。溶剂的这些特性使可逆的 ICA 反应成为可能,还有助于将微尺寸 SnS2 颗粒转化为 3D 多孔纳米结构,同时形成最少的 SEI。我们的 Na–SnS2 半电池在 0.5 C 下的性能达到 1100 mAh g–1(理论容量的 97%),使其成为 Na 存储性能最佳的产品之一。通过超越将电解质溶剂视为离子传输简单介质的传统观点,这项工作强调了溶剂选择对实现 SnS2 阳极的可逆反应和形态转变的关键影响,同时形成最小的 SEI,并为基于 ICA 反应的储能系统中的阳极性能设定了基准。






























 京公网安备 11010802027423号
京公网安备 11010802027423号