Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulating the Atomic Active Center by Covalent Organic Framework-Derived Photothermal Nanozyme to Arm Self-Gelling Powder for Bacterial Wound Healing
ACS Nano ( IF 15.8 ) Pub Date : 2024-12-17 , DOI: 10.1021/acsnano.4c13899 Jing Li, Meng Zhang, Yueyue Wang, Wenxin Lv, Ziran Xu, Bibi Wang, Rongqin Huang, Bingbao Mei, Yi Wang
ACS Nano ( IF 15.8 ) Pub Date : 2024-12-17 , DOI: 10.1021/acsnano.4c13899 Jing Li, Meng Zhang, Yueyue Wang, Wenxin Lv, Ziran Xu, Bibi Wang, Rongqin Huang, Bingbao Mei, Yi Wang
|
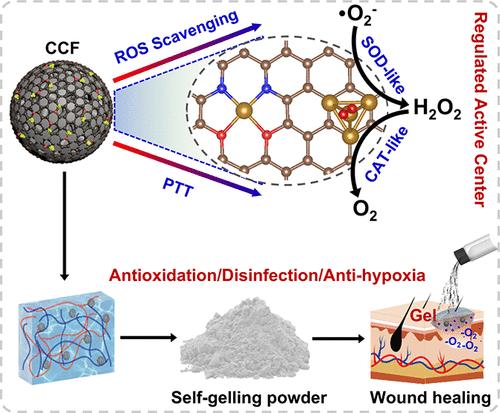
Creating simple methods to produce antioxidant nanozymes with clear structure–activity relationships, particularly aiming to improve disinfection and create practical drug formulations for bacterial wound healing, remains a crucial challenge. Herein, we synthesized iron-loaded covalent organic framework nanospheres, which were then controllably transformed into a carbon-based nanozyme with both iron single atoms and iron clusters through simple pyrolysis. We discovered that the gradual growth of iron clusters significantly boosted the nanozyme’s adsorption onto the substrate and electron transfer, greatly influencing its activity. The nanozyme, optimized by the coexistence of single iron atoms and Fe4 clusters, exhibited the strongest catalase and superoxide dismutase enzyme activities as well as high photothermal efficiency. Under physiological conditions, its peroxidase and oxidase enzymatic activities, which stimulate oxidative stress, remained low. Furthermore, we created an antibacterial self-gelling powder capable of dispersing the nanozyme using polyacrylamide and poly(acrylic acid). The powder can rapidly gel and adhere to wet wound areas, synergistically sterilizing the wound through the combined actions of the gel’s amino groups and the nanozyme’s photothermal effect, while leveraging the antioxidant enzymatic effects to mitigate wound inflammation. These properties contribute to the fast healing of infectious wounds, thus promising a clear formulation and treatment.
中文翻译:

通过共价有机框架衍生的光热纳米酶调节原子活性中心,使自胶凝粉用于细菌伤口愈合
创建简单的方法来生产具有明确结构-活性关系的抗氧化纳米酶,特别是旨在改善消毒和创造用于细菌伤口愈合的实用药物配方,仍然是一项关键挑战。在此,我们合成了负载铁的共价有机框架纳米球,然后通过简单的热解可控地转化为具有铁单原子和铁簇的碳基纳米酶。我们发现,铁簇的逐渐生长显着促进了纳米酶对底物的吸附和电子转移,极大地影响了其活性。通过单个铁原子和 Fe4 簇共存优化的纳米酶表现出最强的过氧化氢酶和超氧化物歧化酶活性以及高光热效率。在生理条件下,其刺激氧化应激的过氧化物酶和氧化酶酶活性保持较低水平。此外,我们创造了一种抗菌自胶凝粉末,能够使用聚丙烯酰胺和聚丙烯酸分散纳米酶。该粉末可以迅速凝胶并粘附在潮湿的伤口区域,通过凝胶的氨基和纳米酶的光热效应的共同作用对伤口进行协同消毒,同时利用抗氧化酶作用来减轻伤口炎症。这些特性有助于感染性伤口的快速愈合,从而有望获得明确的配方和治疗。
更新日期:2024-12-18
中文翻译:

通过共价有机框架衍生的光热纳米酶调节原子活性中心,使自胶凝粉用于细菌伤口愈合
创建简单的方法来生产具有明确结构-活性关系的抗氧化纳米酶,特别是旨在改善消毒和创造用于细菌伤口愈合的实用药物配方,仍然是一项关键挑战。在此,我们合成了负载铁的共价有机框架纳米球,然后通过简单的热解可控地转化为具有铁单原子和铁簇的碳基纳米酶。我们发现,铁簇的逐渐生长显着促进了纳米酶对底物的吸附和电子转移,极大地影响了其活性。通过单个铁原子和 Fe4 簇共存优化的纳米酶表现出最强的过氧化氢酶和超氧化物歧化酶活性以及高光热效率。在生理条件下,其刺激氧化应激的过氧化物酶和氧化酶酶活性保持较低水平。此外,我们创造了一种抗菌自胶凝粉末,能够使用聚丙烯酰胺和聚丙烯酸分散纳米酶。该粉末可以迅速凝胶并粘附在潮湿的伤口区域,通过凝胶的氨基和纳米酶的光热效应的共同作用对伤口进行协同消毒,同时利用抗氧化酶作用来减轻伤口炎症。这些特性有助于感染性伤口的快速愈合,从而有望获得明确的配方和治疗。






























 京公网安备 11010802027423号
京公网安备 11010802027423号