当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Safe and stable Zn-lignin batteries with a biopolymer based hydrogel electrolyte
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-12-18 , DOI: 10.1039/d4ta07213h Ujwala Ail, Jakob Backe, Zia Ullah Khan, Rui Shu, Jaywant Phopase, Magnus Berggren, Reverant Crispin
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-12-18 , DOI: 10.1039/d4ta07213h Ujwala Ail, Jakob Backe, Zia Ullah Khan, Rui Shu, Jaywant Phopase, Magnus Berggren, Reverant Crispin
|
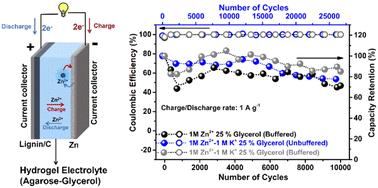
The safety risks associated with organic solvent-based batteries for stationary energy storage have driven scientists to reconsider aqueous electrolytes combined with ultra low-cost materials. In this context, zinc (Zn) metal and biopolymer lignin are certainly among the most abundant and economical electroactive materials on Earth, displaying compatibility in their redox activity to fit the stability window of aqueous electrolytes. But, up to now, the electrolyte solutions in those systems incorporate fluorinated organic salts or bio-ionic liquids, both of which are detrimental to the environment and expensive. In this work we use a state-of-the-art lignin electrode based on catechol functionalized lignin (LC) nano-composited with carbon black (C) and a biopolymer hydrogel electrolyte based on agarose with non-fluorinated Zn salt. The optimization of the hydrogel's composition was realized by reducing the amount of free water by promoting its bonding with additional glycerol. The hydrogel facilitates the growth of Zn in the (002) plane, preventing dendritic formation. The highest discharge capacity of 79.7 mA h gLC−1 was obtained at 0.05 A g−1 charge/discharge rate for the buffered 3% agarose hydrogel electrolyte containing 25% glycerol with 1 M Zn2+. The hydrogel containing 25% glycerol with 1 M Zn2+ and 1 M K+ in the absence of buffering shows the best cycle performance with 78% capacity retention after 26 000 cycles at 1 A g−1 with a capacity of 58 mA h gLC−1 at 0.05 A g−1. This study shows the possibility of a safe, affordable, bio-based environmentally friendly energy storage system that has the potential for large-scale applications.
中文翻译:

采用生物聚合物基水凝胶电解质的安全稳定的 Zn-木质素电池
用于固定储能的有机溶剂型电池存在安全风险,这促使科学家们重新考虑将水性电解质与超低成本材料相结合。在这种情况下,锌 (Zn) 金属和生物聚合物木质素无疑是地球上最丰富和最经济的电活性材料之一,它们的氧化还原活性显示出相容性,以适应水性电解质的稳定性窗口。但是,到目前为止,这些系统中的电解质溶液包含氟化有机盐或生物离子液体,这两种物质都对环境有害且价格昂贵。在这项工作中,我们使用了基于儿茶酚官能化木质素 (LC) 与炭黑 (C) 纳米复合的最先进的木质素电极和基于琼脂糖与非氟化锌盐的生物聚合物水凝胶电解质。水凝胶成分的优化是通过促进游离水与额外甘油的键合来减少游离水的量来实现的。水凝胶促进 Zn 在 (002) 平面中的生长,防止树突状形成。对于含有 25% 甘油和 1 M Zn2+ 的缓冲 3% 琼脂糖水凝胶电解质,在 0.05 A g-1 充电/放电速率下获得 79.7 mA h gLC-1 的最高放电容量。在没有缓冲的情况下,含有 25% 甘油、1 M Zn2+ 和 1 M K+ 的水凝胶在 1 A g-1 下循环 26 000 次后显示出最佳的循环性能,容量保持率为 78%,在 0.05 A g-1 下容量为 58 mA h gLC-1。 这项研究表明,一种安全、经济、生物基的环保储能系统具有大规模应用的潜力。
更新日期:2024-12-18
中文翻译:

采用生物聚合物基水凝胶电解质的安全稳定的 Zn-木质素电池
用于固定储能的有机溶剂型电池存在安全风险,这促使科学家们重新考虑将水性电解质与超低成本材料相结合。在这种情况下,锌 (Zn) 金属和生物聚合物木质素无疑是地球上最丰富和最经济的电活性材料之一,它们的氧化还原活性显示出相容性,以适应水性电解质的稳定性窗口。但是,到目前为止,这些系统中的电解质溶液包含氟化有机盐或生物离子液体,这两种物质都对环境有害且价格昂贵。在这项工作中,我们使用了基于儿茶酚官能化木质素 (LC) 与炭黑 (C) 纳米复合的最先进的木质素电极和基于琼脂糖与非氟化锌盐的生物聚合物水凝胶电解质。水凝胶成分的优化是通过促进游离水与额外甘油的键合来减少游离水的量来实现的。水凝胶促进 Zn 在 (002) 平面中的生长,防止树突状形成。对于含有 25% 甘油和 1 M Zn2+ 的缓冲 3% 琼脂糖水凝胶电解质,在 0.05 A g-1 充电/放电速率下获得 79.7 mA h gLC-1 的最高放电容量。在没有缓冲的情况下,含有 25% 甘油、1 M Zn2+ 和 1 M K+ 的水凝胶在 1 A g-1 下循环 26 000 次后显示出最佳的循环性能,容量保持率为 78%,在 0.05 A g-1 下容量为 58 mA h gLC-1。 这项研究表明,一种安全、经济、生物基的环保储能系统具有大规模应用的潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号