当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mesenchymal Stem Cells‐Derived Small Extracellular Vesicles and Their Validation as a Promising Treatment for Chondrosarcoma in a 3D Model in Vitro
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2024-12-18 , DOI: 10.1002/bit.28909 Eugenia Romano, Francesca Perut, Sofia Avnet, Gemma Di Pompo, Simona Silvestri, Felicia Roffo, Nicola Baldini, Paolo Antonio Netti, Enza Torino
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2024-12-18 , DOI: 10.1002/bit.28909 Eugenia Romano, Francesca Perut, Sofia Avnet, Gemma Di Pompo, Simona Silvestri, Felicia Roffo, Nicola Baldini, Paolo Antonio Netti, Enza Torino
|
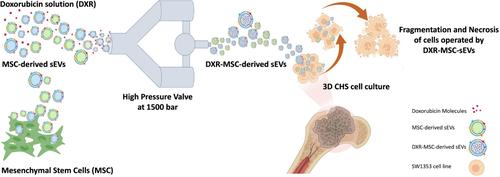
Chondrosarcomas (CHS) constitute approximately 20% of all primary malignant bone tumors, characterized by a slow growth rate with initial manifestation of few signs and symptoms. These malignant cartilaginous neoplasms, particularly those with dedifferentiated histological subtypes, pose significant therapeutic challenges, as they exhibit high resistance to both radiation and chemotherapy. Ranging from relatively benign, low‐grade tumors (grade I) to aggressive high‐grade tumors with the potential for lung metastases and a grim prognosis, there is a critical need for innovative diagnostic and therapeutic approaches, particularly for patients with more aggressive forms. Herein, small extracellular vesicles (sEVs) derived from mesenchymal stem cells are presented as an efficient nanodelivery tool to enhance drug penetration in an in vitro 3D model of CHS. Employing high‐pressure homogenization (HPH), we achieved unprecedented encapsulation efficiency of doxorubicin (DXR) in sEVs derived from mesenchymal stem cells (MSC‐EVs). Subsequently, a comparative analysis between free DXR and MSC‐EVs encapsulated with DXR (DXR‐MSC‐EVs) was conducted to assess their penetration and uptake efficacy in the 3D model. The results unveiled a higher incidence of necrotic cells and a more pronounced toxic effect with DXR‐MSC‐EVs compared to DXR alone. This underscores the remarkable ability of MSC‐EVs to deliver drugs in complex environments, highlighting their potential application in the treatment of aggressive CHS.
中文翻译:

间充质干细胞来源的细胞外小囊泡及其在体外 3D 模型中作为软骨肉瘤的有前途的治疗方法的验证
软骨肉瘤 (CHS) 约占所有原发性恶性骨肿瘤的 20%,其特征是生长速度缓慢,初始表现很少的体征和症状。这些恶性软骨肿瘤,尤其是那些具有去分化组织学亚型的肿瘤,构成了重大的治疗挑战,因为它们对放疗和化疗都表现出高度耐药性。从相对良性的低级别肿瘤(I 级)到具有肺转移潜力和严峻预后严峻的侵袭性高级别肿瘤,迫切需要创新的诊断和治疗方法,特别是对于更具侵袭性的患者。在此,来自间充质干细胞的小细胞外囊泡 (sEV) 作为一种有效的纳米递送工具,以增强药物在 CHS 体外 3D 模型中的渗透性。采用高压匀浆 (HPH),我们在间充质干细胞 (MSC-EV) 衍生的 sEV 中实现了前所未有的阿霉素 (DXR) 包封效率。随后,对游离 DXR 和用 DXR (DXR-MSC-EV) 封装的 MSC-EV 进行了比较分析,以评估它们在 3D 模型中的渗透和吸收效果。结果显示,与单独使用 DXR 相比,DXR-MSC-EVs 的坏死细胞发生率更高,毒性作用更明显。这强调了 MSC-EV 在复杂环境中递送药物的非凡能力,突出了它们在治疗侵袭性 CHS 方面的潜在应用。
更新日期:2024-12-18
中文翻译:

间充质干细胞来源的细胞外小囊泡及其在体外 3D 模型中作为软骨肉瘤的有前途的治疗方法的验证
软骨肉瘤 (CHS) 约占所有原发性恶性骨肿瘤的 20%,其特征是生长速度缓慢,初始表现很少的体征和症状。这些恶性软骨肿瘤,尤其是那些具有去分化组织学亚型的肿瘤,构成了重大的治疗挑战,因为它们对放疗和化疗都表现出高度耐药性。从相对良性的低级别肿瘤(I 级)到具有肺转移潜力和严峻预后严峻的侵袭性高级别肿瘤,迫切需要创新的诊断和治疗方法,特别是对于更具侵袭性的患者。在此,来自间充质干细胞的小细胞外囊泡 (sEV) 作为一种有效的纳米递送工具,以增强药物在 CHS 体外 3D 模型中的渗透性。采用高压匀浆 (HPH),我们在间充质干细胞 (MSC-EV) 衍生的 sEV 中实现了前所未有的阿霉素 (DXR) 包封效率。随后,对游离 DXR 和用 DXR (DXR-MSC-EV) 封装的 MSC-EV 进行了比较分析,以评估它们在 3D 模型中的渗透和吸收效果。结果显示,与单独使用 DXR 相比,DXR-MSC-EVs 的坏死细胞发生率更高,毒性作用更明显。这强调了 MSC-EV 在复杂环境中递送药物的非凡能力,突出了它们在治疗侵袭性 CHS 方面的潜在应用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号