当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
FTO activates PD-L1 promotes immunosuppression in breast cancer via the m6A/YTHDF3/PDK1 axis under hypoxic conditions
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-12-17 , DOI: 10.1016/j.jare.2024.12.026 Siyu Wang, Xingda Zhang, Quanrun Chen, Hao Wu, Shihan Cao, Shilu Zhao, Guozheng Li, Jianyu Wang, Yajie Gong, Xinheng Wang, Da Pang, Song Gao
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-12-17 , DOI: 10.1016/j.jare.2024.12.026 Siyu Wang, Xingda Zhang, Quanrun Chen, Hao Wu, Shihan Cao, Shilu Zhao, Guozheng Li, Jianyu Wang, Yajie Gong, Xinheng Wang, Da Pang, Song Gao
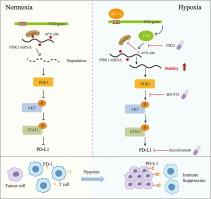
|
Altered epigenetic reprogramming enables breast cancer cells to adapt to hypoxic stress. Hypoxic microenvironment can alter immune cell infiltration and function, limiting the effectiveness of immunotherapy.
中文翻译:

在缺氧条件下,FTO 激活 PD-L1 通过 m6A/YTHDF3/PDK1 轴促进乳腺癌的免疫抑制
改变的表观遗传重编程使乳腺癌细胞能够适应低氧应激。缺氧微环境会改变免疫细胞的浸润和功能,从而限制免疫治疗的有效性。
更新日期:2024-12-17
中文翻译:

在缺氧条件下,FTO 激活 PD-L1 通过 m6A/YTHDF3/PDK1 轴促进乳腺癌的免疫抑制
改变的表观遗传重编程使乳腺癌细胞能够适应低氧应激。缺氧微环境会改变免疫细胞的浸润和功能,从而限制免疫治疗的有效性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号