当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biomimetic Synthesis of Azorellolide via Cyclopropylcarbinyl Cation Chemistry
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-18 , DOI: 10.1021/jacs.4c14664 Jordan Y. Artzy, Dean J. Tantillo, Dirk H. Trauner
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-18 , DOI: 10.1021/jacs.4c14664 Jordan Y. Artzy, Dean J. Tantillo, Dirk H. Trauner
|
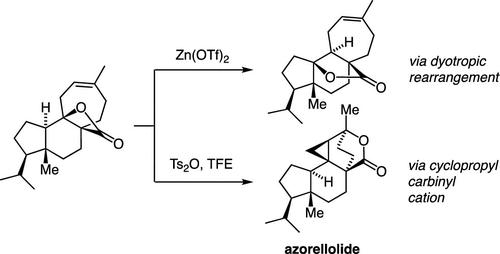
A concise synthesis of the complex diterpene azorellolide, inspired by speculations on biosynthetic cationic cascades, is presented. The approach, guided by computation, relies on the intramolecular interception of a cyclopropylcarbinyl cation by an appended carboxylate. The successful execution of this strategy was achieved through acid-catalyzed isomerization of a β-lactone in competition with a type I dyotropic rearrangement.
中文翻译:

通过环丙基甲酰阳离子化学仿生合成 Azorellolide
提出了络合二萜氮杂内酯的简明合成,其灵感来自对生物合成阳离子级联反应的推测。该方法以计算为指导,依赖于附加的羧酸盐对环丙基甲酰阳离子的分子内拦截。该策略的成功执行是通过 β-内酯的酸催化异构化与 I 型二嗜性重排竞争来实现的。
更新日期:2024-12-18
中文翻译:

通过环丙基甲酰阳离子化学仿生合成 Azorellolide
提出了络合二萜氮杂内酯的简明合成,其灵感来自对生物合成阳离子级联反应的推测。该方法以计算为指导,依赖于附加的羧酸盐对环丙基甲酰阳离子的分子内拦截。该策略的成功执行是通过 β-内酯的酸催化异构化与 I 型二嗜性重排竞争来实现的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号