当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substituent Effects on Cooperativity in Three-Component H-Bond Networks Involving Phenol–Phenol Interactions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-18 , DOI: 10.1021/jacs.4c15767 Lucia Trevisan, Andrew D. Bond, Christopher A. Hunter
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-18 , DOI: 10.1021/jacs.4c15767 Lucia Trevisan, Andrew D. Bond, Christopher A. Hunter
|
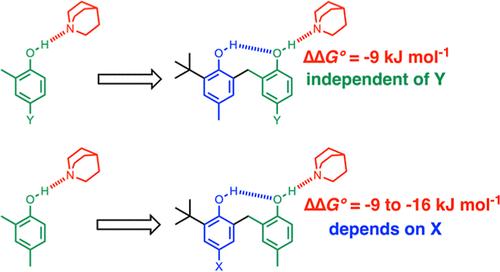
Cooperativity between H-bonding interactions in networks is a fundamental aspect of solvation and self-assembly in molecular systems. The interaction of a series of bisphenols, which make an intramolecular H-bond between the two hydroxyl groups, and quinuclidine was used to quantify cooperativity in three-component networks. The presence of the intramolecular H-bond in the bisphenols was established by using 1H NMR spectroscopy in solution and X-ray crystallography in the solid state. The interactions with quinuclidine were investigated using UV–vis and 1H NMR titrations, which show that the intramolecular hydrogen bonds persist in the 1:1 complexes. By varying substituents on one of the phenol groups, it was possible to measure the effect of changing the strength of the intramolecular H-bond between the hydroxyl groups on the strength of the intermolecular H-bond with quinuclidine. Strong positive cooperativity was observed between the two interactions, with increases in binding free energy of up to 16 kJ mol–1. By varying substituents on the other phenol group, which makes both an intramolecular H-bond and an intermolecular H-bond in the complex, it was possible to measure how the properties of this central hydroxyl group modulate cooperativity between the interactions with the other two functional groups. Changing the polarity of this phenol had no effect on the measured cooperativity. The results indicate that cooperativity in H-bond networks can be understood as a polar interaction between two remote functional groups that is damped by a central functional group. The extent of damping is quantified by cooperativity parameter κ, which is 0.33 for the hydroxyl group and appears to be an intrinsic property of the geometry or polarizability of the functional group rather than polarity.
中文翻译:

取代基对涉及苯酚-苯酚相互作用的三组分 H-键网络中的协同性的影响
网络中 H 键相互作用之间的协同性是分子系统中溶剂化和自组装的一个基本方面。一系列双酚(在两个羟基之间形成分子内 H 键)与喹啉的相互作用用于量化三组分网络中的协同性。通过在溶液中使用 1 H NMR 波谱和在固态中使用 X射线晶体学来确定双酚中分子内 H 键的存在。使用 UV-vis 和 1H NMR 滴定研究与喹啉啶的相互作用,结果表明分子内氢键在 1:1 复合物中持续存在。通过改变其中一个酚基上的取代基,可以测量改变羟基之间分子内 H 键强度对喹啉的分子间 H 键强度的影响。在两种相互作用之间观察到很强的正协同性,结合自由能增加高达 16 kJ mol–1。通过改变另一个酚基上的取代基,在复合物中形成分子内 H 键和分子间 H 键,可以测量该中心羟基的性质如何调节与其他两个官能团相互作用之间的协同性。改变这种苯酚的极性对测得的协同性没有影响。结果表明,H键网络中的协同性可以理解为两个远程官能团之间的极性相互作用,它被一个中心官能团阻尼。阻尼的程度由协同性参数 κ 量化,该参数为 0。33 对于羟基,似乎是官能团的几何形状或极化率的固有性质,而不是极性。
更新日期:2024-12-18
中文翻译:

取代基对涉及苯酚-苯酚相互作用的三组分 H-键网络中的协同性的影响
网络中 H 键相互作用之间的协同性是分子系统中溶剂化和自组装的一个基本方面。一系列双酚(在两个羟基之间形成分子内 H 键)与喹啉的相互作用用于量化三组分网络中的协同性。通过在溶液中使用 1 H NMR 波谱和在固态中使用 X射线晶体学来确定双酚中分子内 H 键的存在。使用 UV-vis 和 1H NMR 滴定研究与喹啉啶的相互作用,结果表明分子内氢键在 1:1 复合物中持续存在。通过改变其中一个酚基上的取代基,可以测量改变羟基之间分子内 H 键强度对喹啉的分子间 H 键强度的影响。在两种相互作用之间观察到很强的正协同性,结合自由能增加高达 16 kJ mol–1。通过改变另一个酚基上的取代基,在复合物中形成分子内 H 键和分子间 H 键,可以测量该中心羟基的性质如何调节与其他两个官能团相互作用之间的协同性。改变这种苯酚的极性对测得的协同性没有影响。结果表明,H键网络中的协同性可以理解为两个远程官能团之间的极性相互作用,它被一个中心官能团阻尼。阻尼的程度由协同性参数 κ 量化,该参数为 0。33 对于羟基,似乎是官能团的几何形状或极化率的固有性质,而不是极性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号