当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tropospheric Fate of Methylhydroxycarbene and the Ability of a Single Water Molecule to Efficiently Promote Its Isomerization into Acetaldehyde
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-17 , DOI: 10.1021/jacs.4c08903 Soumen Mondal, Saikat Sadhukhan, Amitabha Sinha, Montu K. Hazra
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-17 , DOI: 10.1021/jacs.4c08903 Soumen Mondal, Saikat Sadhukhan, Amitabha Sinha, Montu K. Hazra
|
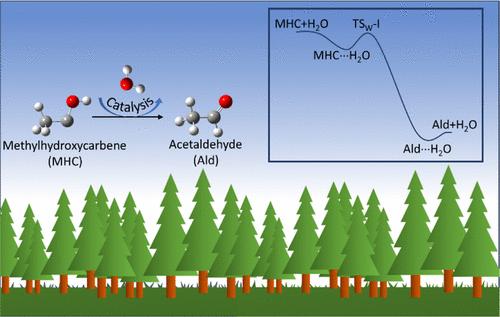
The ultraviolet (UV) photodissociation of pyruvic acid through the absorption of solar actinic flux generates methylhydroxycarbene (MHC) in the atmosphere. It is recognized that isolated MHC can undergo unimolecular isomerization to form acetaldehyde and vinyl alcohol. However, the rates and mechanism for its possible bimolecular reactions with atmospheric constituents, which can occur in parallel with its unimolecular reaction, is not well understood. Here we investigate the energetics, kinetics, and mechanism of the reaction of MHC with three ubiquitous atmospheric molecules N2, O2, and H2O over the 160 K-380 K temperature range. Our study, at the CCSD(T)/6-311++G(3df,3pd)//M06-2X/6-311++G(3df,3pd) level, reveals that the MHC + N2 encounter is nonreactive, while the MHC + O2 reaction, which leads to CH3CO + HO2 formation, has a rate that is significantly different from previous estimates. For the MHC + H2O reaction, we find that a single H2O molecule is very effective in catalyzing the isomerization of MHC to form predominantly acetaldehyde. An analysis of the computed rate for this reaction indicates that it will be an important source of tropospheric acetaldehyde ̵ a major pollutant and precursor for atmospheric reactive intermediates. Our findings are in sharp contrast to current assessments in the literature that the MHC + H2O reaction is minor. Furthermore, in the MHC + H2O reaction system, we find that due to the presence of the OH group on MHC, the concerted insertion mechanism, which is typically dominant in reactions involving singlet carbenes, is suppressed relative to a hydrogen bond mediated double hydrogen atom transfer mechanism.
中文翻译:

甲基羟基卡宾的对流层命运和单个水分子有效促进其异构化为乙醛的能力
丙酮酸通过吸收太阳光化通量进行紫外线 (UV) 光解离,在大气中生成甲基羟基卡宾 (MHC)。人们认识到,分离的 MHC 可以进行单分子异构化以形成乙醛和乙烯醇。然而,它与大气成分可能发生的双分子反应的速率和机制尚不清楚,这可能与其单分子反应同时发生。在这里,我们研究了 MHC 在 160 K-380 K 温度范围内与三种普遍存在的大气分子 N2、O2 和 H2O 反应的能量学、动力学和机理。我们的研究在 CCSD(T)/6-311++G(3df,3pd)//M06-2X/6-311++G(3df,3pd) 水平上显示,MHC + N2 遭遇是非反应性的,而导致 CH3CO + HO2 形成的 MHC + O2 反应的速率与之前的估计显着不同。对于 MHC + H2O 反应,我们发现单个 H2O 分子在催化 MHC 异构化以形成主要乙醛方面非常有效。对该反应的计算速率的分析表明,它将成为对流层乙醛的重要来源,乙醛是大气反应性中间体的主要污染物和前体。我们的研究结果与目前文献中关于 MHC + H2O 反应很小的评估形成鲜明对比。此外,在 MHC + H2O 反应系统中,我们发现由于 MHC 上存在 OH 基团,相对于氢键介导的双氢原子转移机制,通常在涉及单线态卡宾的反应中占主导地位的协同插入机制受到抑制。
更新日期:2024-12-18
中文翻译:

甲基羟基卡宾的对流层命运和单个水分子有效促进其异构化为乙醛的能力
丙酮酸通过吸收太阳光化通量进行紫外线 (UV) 光解离,在大气中生成甲基羟基卡宾 (MHC)。人们认识到,分离的 MHC 可以进行单分子异构化以形成乙醛和乙烯醇。然而,它与大气成分可能发生的双分子反应的速率和机制尚不清楚,这可能与其单分子反应同时发生。在这里,我们研究了 MHC 在 160 K-380 K 温度范围内与三种普遍存在的大气分子 N2、O2 和 H2O 反应的能量学、动力学和机理。我们的研究在 CCSD(T)/6-311++G(3df,3pd)//M06-2X/6-311++G(3df,3pd) 水平上显示,MHC + N2 遭遇是非反应性的,而导致 CH3CO + HO2 形成的 MHC + O2 反应的速率与之前的估计显着不同。对于 MHC + H2O 反应,我们发现单个 H2O 分子在催化 MHC 异构化以形成主要乙醛方面非常有效。对该反应的计算速率的分析表明,它将成为对流层乙醛的重要来源,乙醛是大气反应性中间体的主要污染物和前体。我们的研究结果与目前文献中关于 MHC + H2O 反应很小的评估形成鲜明对比。此外,在 MHC + H2O 反应系统中,我们发现由于 MHC 上存在 OH 基团,相对于氢键介导的双氢原子转移机制,通常在涉及单线态卡宾的反应中占主导地位的协同插入机制受到抑制。






























 京公网安备 11010802027423号
京公网安备 11010802027423号