当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Oxidative Coupling between C(sp3)–H Bonds and Carboxylic Acids
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-17 , DOI: 10.1021/jacs.4c12544 Xian-Ming Liu, Fu Li, Tongkun Wang, Ling Dai, Yin Yang, Neng-Quan Jiang, Li-Yuan Xue, Jing-Yuan Liu, Xiao-Song Xue, Li-Jun Xiao, Qi-Lin Zhou
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-17 , DOI: 10.1021/jacs.4c12544 Xian-Ming Liu, Fu Li, Tongkun Wang, Ling Dai, Yin Yang, Neng-Quan Jiang, Li-Yuan Xue, Jing-Yuan Liu, Xiao-Song Xue, Li-Jun Xiao, Qi-Lin Zhou
|
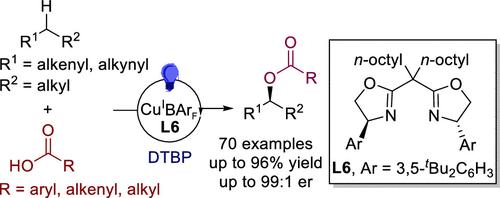
The direct enantioselective functionalization of C(sp3)–H bonds in organic molecules could fundamentally transform the synthesis of chiral molecules. In particular, the enantioselective oxidation of these bonds would dramatically change the production methods of chiral alcohols and esters, which are prevalent in natural products, pharmaceuticals, and fine chemicals. Remarkable advances have been made in the enantioselective construction of carbon–carbon and carbon–nitrogen bonds through the C(sp3)–H bond functionalization. However, the direct enantioselective formation of carbon–oxygen bonds from C(sp3)–H bonds remains a considerable challenge. We herein report a highly enantioselective C(sp3)–H bond oxidative coupling with carboxylic acids. The method applies to allylic and propargylic C–H bonds and employs various carboxylic acids as oxygenating agents. The method successfully synthesized a range of chiral esters directly from readily available alkenes and alkynes, greatly simplifying the synthesis of chiral esters and related alcohols.
中文翻译:

C(sp3)-H 键与羧酸之间的催化不对称氧化偶联
有机分子中 C(sp3)-H 键的直接对映选择性官能团化可以从根本上改变手性分子的合成。特别是,这些键的对映选择性氧化将极大地改变手性醇和酯的生产方法,这些醇和酯在天然产物、药物和精细化学品中普遍存在。通过 C(sp3)-H 键功能化,碳-碳和碳-氮键的对映选择性构建取得了显著进展。然而,从 C(sp3)-H 键直接对映选择性形成碳-氧键仍然是一个相当大的挑战。我们在此报道了一种与羧酸的高对映选择性 C(sp3)-H 键氧化偶联。该方法适用于烯丙基和炔丙基 C-H 键,并采用各种羧酸作为氧化剂。该方法成功地直接从现成的烯烃和炔烃合成了一系列手性酯,大大简化了手性酯和相关醇的合成。
更新日期:2024-12-18
中文翻译:

C(sp3)-H 键与羧酸之间的催化不对称氧化偶联
有机分子中 C(sp3)-H 键的直接对映选择性官能团化可以从根本上改变手性分子的合成。特别是,这些键的对映选择性氧化将极大地改变手性醇和酯的生产方法,这些醇和酯在天然产物、药物和精细化学品中普遍存在。通过 C(sp3)-H 键功能化,碳-碳和碳-氮键的对映选择性构建取得了显著进展。然而,从 C(sp3)-H 键直接对映选择性形成碳-氧键仍然是一个相当大的挑战。我们在此报道了一种与羧酸的高对映选择性 C(sp3)-H 键氧化偶联。该方法适用于烯丙基和炔丙基 C-H 键,并采用各种羧酸作为氧化剂。该方法成功地直接从现成的烯烃和炔烃合成了一系列手性酯,大大简化了手性酯和相关醇的合成。






























 京公网安备 11010802027423号
京公网安备 11010802027423号