当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiplicativity in Solubility Isotherms
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-12-18 , DOI: 10.1021/acs.iecr.4c03215
Seishi Shimizu 1 , Nobuyuki Matubayasi 2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-12-18 , DOI: 10.1021/acs.iecr.4c03215
Seishi Shimizu 1 , Nobuyuki Matubayasi 2
Affiliation
|
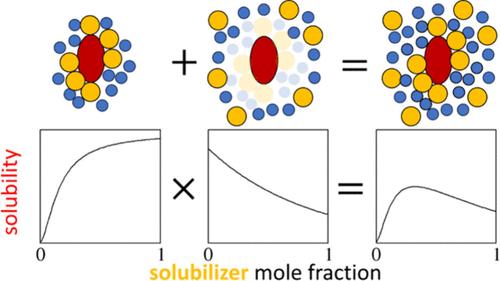
With the help of isotherm equations, how solubility changes with solubilizer concentration (“solubility isotherm”) can reveal the underlying interactions. However, despite their success in elucidating the mechanisms of hydrotropy (via the cooperative (sigmoidal) isotherm) and synergistic solvation (via the quadratic (bell-shaped) isotherm), these simple statistical thermodynamic isotherm equations alone are insufficient for more complex isotherms that combine their features. Here, we show (i) how simple isotherms can be combined via the isotherm multiplicativity rule founded on the excess number relationship (i.e., solubilizer concentration dependence of the solubilizer excess number around a solute) and (ii) how (i) leads to successful modeling of complex solubility isotherms, capturing that cooperative solute–solubilizer association, in turn, induces the exclusion of solubilizers from the already crowded solute’s locality at higher concentrations. Moreover, we will demonstrate that both the cooperative and quadratic solubility isotherms can be derived directly from the excess number relationship, establishing it not only as the basis for the multiplicativity rule but also as the fundamental relationship for simple and complex solubility isotherms.
中文翻译:

溶解度等温线中的倍增性
在等温线方程的帮助下,溶解度如何随增溶剂浓度的变化(“溶解度等温线”)可以揭示潜在的相互作用。然而,尽管它们成功地阐明了水动力(通过协同(S 形)等温线)和协同溶剂化(通过二次(钟形)等温线)的机制,但这些简单的统计热力学等温线方程本身不足以用于结合其特征的更复杂的等温线。在这里,我们展示了 (i) 如何通过基于超量数关系的等温线乘数规则(即增溶剂浓度对溶质周围增溶剂超量数的依赖性)组合简单等温线,以及 (ii) (i) 如何成功模拟复溶性等温线,捕获协作溶质-增溶剂结合,反过来,诱导增溶剂从较高浓度下已经拥挤的溶质位置中排除。此外,我们将证明合作溶解度等温线和二次溶解度等温线都可以直接从超数关系中得出,不仅将其确立为乘法规则的基础,而且作为简单和复杂溶解度等温线的基本关系。
更新日期:2024-12-18
中文翻译:

溶解度等温线中的倍增性
在等温线方程的帮助下,溶解度如何随增溶剂浓度的变化(“溶解度等温线”)可以揭示潜在的相互作用。然而,尽管它们成功地阐明了水动力(通过协同(S 形)等温线)和协同溶剂化(通过二次(钟形)等温线)的机制,但这些简单的统计热力学等温线方程本身不足以用于结合其特征的更复杂的等温线。在这里,我们展示了 (i) 如何通过基于超量数关系的等温线乘数规则(即增溶剂浓度对溶质周围增溶剂超量数的依赖性)组合简单等温线,以及 (ii) (i) 如何成功模拟复溶性等温线,捕获协作溶质-增溶剂结合,反过来,诱导增溶剂从较高浓度下已经拥挤的溶质位置中排除。此外,我们将证明合作溶解度等温线和二次溶解度等温线都可以直接从超数关系中得出,不仅将其确立为乘法规则的基础,而且作为简单和复杂溶解度等温线的基本关系。

































 京公网安备 11010802027423号
京公网安备 11010802027423号