当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of EDTA and Bicarbonate on U(VI) Reduction by Reduced Nontronite
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-12-18 , DOI: 10.1021/acs.est.4c09492 Shuaidi Wang, Yu Chen, Zezhen Pan, Juan Liu, Yuefei Ding, Yuheng Wang, Dong Liu, Songlin Wu, Dafu Hu, Runjie Li, Qingyin Xia, Limin Zhang, Hailiang Dong
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-12-18 , DOI: 10.1021/acs.est.4c09492 Shuaidi Wang, Yu Chen, Zezhen Pan, Juan Liu, Yuefei Ding, Yuheng Wang, Dong Liu, Songlin Wu, Dafu Hu, Runjie Li, Qingyin Xia, Limin Zhang, Hailiang Dong
|
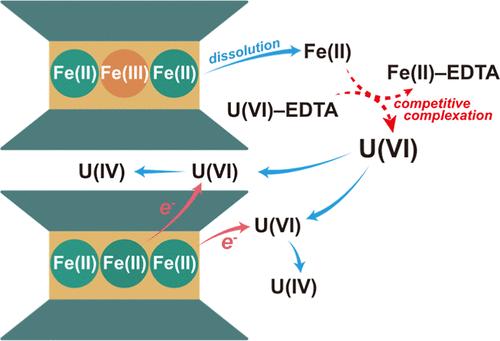
Widespread Fe-bearing clay minerals are potential materials capable of reducing and immobilizing U(VI). However, the kinetics of this process and the impact of environmental factors remain unclear. Herein, we investigated U(VI) reduction by chemically reduced nontronite (rNAu-2) in the presence of EDTA and bicarbonate. U(VI) was completely reduced within 192 h by rNAu-2 alone, and higher Fe(II) in rNAu-2 resulted in a higher U(VI) reduction rate. However, the presence of EDTA and NaHCO3 initially inhibited U(VI) reduction by forming stable U(VI)–EDTA/carbonato complexes and thus preventing U(VI) from adsorbing onto the rNAu-2 surface. However, over time, EDTA facilitated the dissolution of rNAu-2, releasing Fe(II) into solution. Released Fe(II) competed with U(VI) to form Fe(II)–EDTA complexes, thus freeing U(VI) from negatively charged U(VI)–EDTA complexes to form positively charged U(VI)–OH complexes, which ultimately promoted U(VI) adsorption and triggered its reduction. In the NaHCO3 system, U(VI) complexed with carbonate to form U(VI)–carbonato complexes, which partially inhibited adsorption to the rNAu-2 surface and subsequent reduction. The reduced U(IV) largely formed uraninite nanoparticles, with a fraction present in the rNAu-2 interlayer. Our results demonstrate the important impacts of clay minerals, organic matter, and bicarbonate on U(VI) reduction, providing crucial insights into the uranium biogeochemistry in the subsurface environment and remediation strategies for uranium-contaminated environments.
中文翻译:

EDTA 和碳酸氢盐对还原非特罗石减少 U(VI) 的影响
广泛分布的含铁粘土矿物是能够还原和固定 U(VI) 的潜在材料。然而,这个过程的动力学和环境因素的影响仍不清楚。在此,我们研究了在 EDTA 和碳酸氢盐存在下通过化学还原的非子酸盐 (rNAu-2) 还原 U(VI)。U(VI) 在 192 小时内被单独的 rNAu-2 完全还原,并且 rNAu-2 中较高的 Fe(II) 导致较高的 U(VI) 还原率。然而,EDTA 和 NaHCO3 的存在最初通过形成稳定的 U(VI)-EDTA/carbonato 复合物来抑制 U(VI) 还原,从而阻止 U(VI) 吸附到 rNAu-2 表面。然而,随着时间的推移,EDTA 促进了 rNAu-2 的溶解,将 Fe(II) 释放到溶液中。释放的 Fe(II) 与 U(VI) 竞争形成 Fe(II)-EDTA 配合物,从而将 U(VI) 从带负电荷的 U(VI)-EDTA 配合物中释放出来,形成带正电荷的 U(VI)-OH 配合物,最终促进 U(VI) 吸附并触发其还原。在 NaHCO3 系统中,U(VI) 与碳酸盐络合形成 U(VI)-碳酸盐络合物,部分抑制了对 rNAu-2 表面的吸附和随后的还原。还原的 U(IV) 主要形成铀酸盐纳米颗粒,其中一部分存在于 rNAu-2 夹层中。我们的结果证明了粘土矿物、有机物和碳酸氢盐对 U(VI) 还原的重要影响,为地下环境中的铀生物地球化学和铀污染环境的修复策略提供了重要见解。
更新日期:2024-12-18
中文翻译:

EDTA 和碳酸氢盐对还原非特罗石减少 U(VI) 的影响
广泛分布的含铁粘土矿物是能够还原和固定 U(VI) 的潜在材料。然而,这个过程的动力学和环境因素的影响仍不清楚。在此,我们研究了在 EDTA 和碳酸氢盐存在下通过化学还原的非子酸盐 (rNAu-2) 还原 U(VI)。U(VI) 在 192 小时内被单独的 rNAu-2 完全还原,并且 rNAu-2 中较高的 Fe(II) 导致较高的 U(VI) 还原率。然而,EDTA 和 NaHCO3 的存在最初通过形成稳定的 U(VI)-EDTA/carbonato 复合物来抑制 U(VI) 还原,从而阻止 U(VI) 吸附到 rNAu-2 表面。然而,随着时间的推移,EDTA 促进了 rNAu-2 的溶解,将 Fe(II) 释放到溶液中。释放的 Fe(II) 与 U(VI) 竞争形成 Fe(II)-EDTA 配合物,从而将 U(VI) 从带负电荷的 U(VI)-EDTA 配合物中释放出来,形成带正电荷的 U(VI)-OH 配合物,最终促进 U(VI) 吸附并触发其还原。在 NaHCO3 系统中,U(VI) 与碳酸盐络合形成 U(VI)-碳酸盐络合物,部分抑制了对 rNAu-2 表面的吸附和随后的还原。还原的 U(IV) 主要形成铀酸盐纳米颗粒,其中一部分存在于 rNAu-2 夹层中。我们的结果证明了粘土矿物、有机物和碳酸氢盐对 U(VI) 还原的重要影响,为地下环境中的铀生物地球化学和铀污染环境的修复策略提供了重要见解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号