当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering oxygen vacancies in acid-etched MgMn2O4 for efficiently catalytic benzene combustion: Synergistic activation of gaseous oxygen and surface lattice oxygen
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-18 , DOI: 10.1016/j.jhazmat.2024.136907 Yu Wu, Dongjing Lei, Aijie Wang, Qiuyan Zhang, Hongwei Jian, Haojie Yang, Chong Han
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-18 , DOI: 10.1016/j.jhazmat.2024.136907 Yu Wu, Dongjing Lei, Aijie Wang, Qiuyan Zhang, Hongwei Jian, Haojie Yang, Chong Han
|
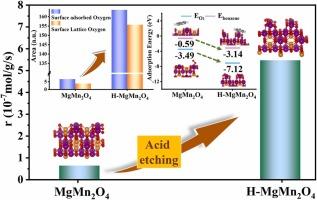
The synergistic activation of gaseous oxygen and surface lattice oxygen is essential for designing highly efficient catalysts to eliminate VOCs. Herein, an effective acid treatment was carried out to create more oxygen vacancies by modulating the electronic structure of MgMn2O4 spinels and MgMnOx mixed oxides. The acid-treated MgMn2O4 exhibited outstanding catalytic performance, with the reaction rate of benzene rising by 8.55 times at 200 °C. After acid treatment, MgMn2O4 partially retained its spinel structure, while Mn2O3 in situ grew on the surface due to the selective removal of Mg2+. The transformation of Mn–O–Mg into Mn–O weakened the strength of adjacent Mn–O bonds, thereby promoting the release of surface lattice oxygen and the regeneration of oxygen vacancies. In addition, acid-treated MgMn2O4 facilitated the adsorption and activation of gaseous oxygen. In situ DRIFTS analysis proved that the synergistic activation of gaseous oxygen and surface lattice oxygen accelerated the conversion of intermediates, thus contributing to the efficient degradation of benzene.
中文翻译:

酸蚀 MgMn2O4 中的工程氧空位用于高效催化苯燃烧:气态氧和表面晶格氧的协同活化
气态氧和表面晶格氧的协同活化对于设计高效催化剂以消除 VOC 至关重要。在此,进行了有效的酸处理,通过调节 MgMn 2 O 4 尖晶石和 MgMnO x 混合氧化物的电子结构来产生更多的氧空位。酸处理后的 MgMn 2 O 4 表现出优异的催化性能,在 200 °C 时苯的反应速率提高了 8.55 倍。 经过酸处理后,MgMn 2 O 4 部分保留了其尖晶石结构,而由于 Mg 2+ 的选择性去除,Mn 2 O 3 原位生长在表面。Mn-O-Mg 向 Mn-O 的转变削弱了相邻 Mn-O 键的强度,从而促进了表面晶格氧的释放和氧空位的再生。此外,酸处理的 MgMn 2 O 4 促进了气态氧的吸附和活化。原位 DRIFTS 分析证明,气态氧和表面晶格氧的协同活化加速了中间体的转化,从而有助于苯的高效降解。
更新日期:2024-12-18
中文翻译:

酸蚀 MgMn2O4 中的工程氧空位用于高效催化苯燃烧:气态氧和表面晶格氧的协同活化
气态氧和表面晶格氧的协同活化对于设计高效催化剂以消除 VOC 至关重要。在此,进行了有效的酸处理,通过调节 MgMn 2 O 4 尖晶石和 MgMnO x 混合氧化物的电子结构来产生更多的氧空位。酸处理后的 MgMn 2 O 4 表现出优异的催化性能,在 200 °C 时苯的反应速率提高了 8.55 倍。 经过酸处理后,MgMn 2 O 4 部分保留了其尖晶石结构,而由于 Mg 2+ 的选择性去除,Mn 2 O 3 原位生长在表面。Mn-O-Mg 向 Mn-O 的转变削弱了相邻 Mn-O 键的强度,从而促进了表面晶格氧的释放和氧空位的再生。此外,酸处理的 MgMn 2 O 4 促进了气态氧的吸附和活化。原位 DRIFTS 分析证明,气态氧和表面晶格氧的协同活化加速了中间体的转化,从而有助于苯的高效降解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号