当前位置:
X-MOL 学术
›
J. Clean. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Constructing electron-rich metal sites in M0.5Co0.5O through N substitution for efficient peroxymonosulfate activation to degrade organic pollutants
Journal of Cleaner Production ( IF 9.7 ) Pub Date : 2024-12-18 , DOI: 10.1016/j.jclepro.2024.144497 Hui Cui, Chuanhui Wang, Yaqi Huang, Mengjie Qin, Ding Zhao, Xianfeng Yang, Peng Guo, Yuanyuan Sun, Dongjiang Yang
Journal of Cleaner Production ( IF 9.7 ) Pub Date : 2024-12-18 , DOI: 10.1016/j.jclepro.2024.144497 Hui Cui, Chuanhui Wang, Yaqi Huang, Mengjie Qin, Ding Zhao, Xianfeng Yang, Peng Guo, Yuanyuan Sun, Dongjiang Yang
|
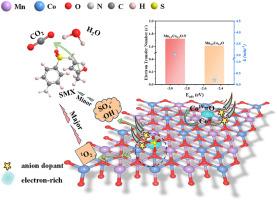
Transition metal oxides are promising heterogeneous catalysts for peroxymonosulfate (PMS) activation. However, the catalytic degradation performance was unsatisfactory. Herein, nitrogen doping was applied to construct electron-rich metal sites in bimetallic oxides (Mn0.5 Co0.5 O, Fe0.5 Co0.5 O, Cu0.5 Co0.5 O) to boost their PMS activation performance for sulfamethoxazole (SMX) degradation. The N-doped bimetallic oxides (Mn0.5 Co0.5 O-N, Fe0.5 Co0.5 O-N, Cu0.5 Co0.5 O-N), obtaining through a facile ammonia-assisted medium-temperature heat treatment method, displayed enhanced PMS activation performance for SMX degradation compared with the pristine bimetallic oxides. Especially, Mn0.5 Co0.5 O-N was the optimal option with 100% SMX degradation efficiency within 2 min, wide pH application range (3.5–11.5), and excellent cycling performance. The density functional theory (DFT) calculations confirmed that Mn0.5 Co0.5 O-N with more negative adsorption energy (Eads ) and higher electron transfer number was more beneficial for PMS adsorption and activation. Quenching experiments, electron paramagnetic resonance (EPR), and solvent exchange (H2 O to D2 O) indicated that 1 O2 contributed predominantly to SMX degradation. This research offers an economical strategy for boosting the PMS activation activity to degrade pollutants of transition metal oxides through constructing electron-rich metal sites in bimetallic oxides by N substitution.
中文翻译:

通过 N 取代在 M0.5Co0.5O 中构建富电子金属位点,以实现高效的过氧一硫酸盐活化以降解有机污染物
过渡金属氧化物是有前途的过氧一硫酸盐 (PMS) 活化异相催化剂。然而,催化降解性能并不令人满意。本文采用氮掺杂在双金属氧化物 (Mn0.5Co0.5O、Fe0.5Co0.5O、Cu0.5Co0.5O) 中构建富电子金属位点,以提高其 PMS 活化性能,用于磺胺甲噁唑 (SMX) 降解。通过简单的氨辅助中温热处理方法获得的 N 掺杂双金属氧化物 (Mn0.5Co0.5O-N、Fe0.5Co0.5O-N、Cu0.5Co0.5O-N) 与原始双金属氧化物相比,显示出增强的 PMS 活化性能,用于 SMX 降解。特别是,Mn0.5Co0.5O-N 是最佳选择,在 2 分钟内 SMX 降解效率达到 100%,pH 应用范围广 (3.5–11.5),循环性能优异。密度泛函理论 (DFT) 计算证实,负吸附能 (Eads) 较高、电子转移数较高的 Mn0.5Co0.5O-N 更有利于 PMS 的吸附和活化。淬灭实验、电子顺磁共振 (EPR) 和溶剂交换 (H2O 到 D2O) 表明 1O2 主要导致 SMX 降解。这项研究提供了一种经济的策略,通过氮取代在双金属氧化物中构建富电子金属位点,从而提高 PMS 活化活性以降解过渡金属氧化物的污染物。
更新日期:2024-12-18
中文翻译:

通过 N 取代在 M0.5Co0.5O 中构建富电子金属位点,以实现高效的过氧一硫酸盐活化以降解有机污染物
过渡金属氧化物是有前途的过氧一硫酸盐 (PMS) 活化异相催化剂。然而,催化降解性能并不令人满意。本文采用氮掺杂在双金属氧化物 (Mn0.5Co0.5O、Fe0.5Co0.5O、Cu0.5Co0.5O) 中构建富电子金属位点,以提高其 PMS 活化性能,用于磺胺甲噁唑 (SMX) 降解。通过简单的氨辅助中温热处理方法获得的 N 掺杂双金属氧化物 (Mn0.5Co0.5O-N、Fe0.5Co0.5O-N、Cu0.5Co0.5O-N) 与原始双金属氧化物相比,显示出增强的 PMS 活化性能,用于 SMX 降解。特别是,Mn0.5Co0.5O-N 是最佳选择,在 2 分钟内 SMX 降解效率达到 100%,pH 应用范围广 (3.5–11.5),循环性能优异。密度泛函理论 (DFT) 计算证实,负吸附能 (Eads) 较高、电子转移数较高的 Mn0.5Co0.5O-N 更有利于 PMS 的吸附和活化。淬灭实验、电子顺磁共振 (EPR) 和溶剂交换 (H2O 到 D2O) 表明 1O2 主要导致 SMX 降解。这项研究提供了一种经济的策略,通过氮取代在双金属氧化物中构建富电子金属位点,从而提高 PMS 活化活性以降解过渡金属氧化物的污染物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号